Abstract
In recent years, 47Sc has attracted attention because of its favorable decay characteristics (half-life, 3.35 d; average energy, 162 keV; Eγ, 159 keV) for therapeutic application and for SPECT imaging. The aim of the present study was to investigate the suitability of 47Sc for radionuclide therapy in a preclinical setting. For this purpose a novel DOTA-folate conjugate (cm10) with an albumin-binding entity was used. Methods: 47Sc was produced via the 46Ca(n,γ)47Ca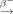 47Sc nuclear reaction at the high-flux reactor at the Institut Laue-Langevin. Separation of the 47Sc from the target material was performed by a semi-automated process using extraction chromatography and cation exchange chromatography. 47Sc-labeled cm10 was tested on folate receptor–positive KB tumor cells in vitro. Biodistribution and SPECT imaging experiments were performed in KB tumor–bearing mice. Radionuclide therapy was conducted with two groups of mice, which received either 47Sc-cm10 (10 MBq) or only saline. Tumor growth and survival time were compared between the two groups of mice. Results: Irradiation of 46Ca resulted in approximately 1.8 GBq of 47Ca, which subsequently decayed to 47Sc. Separation of 47Sc from 47Ca was obtained with 80% yield in only 10 min. The 47Sc was then available in a small volume (∼500 μL) of an ammonium acetate/HCl (pH 4.5) solution suitable for direct radiolabeling. 47Sc-cm10 was prepared with a radiochemical yield of more than 96% at a specific activity of up to 13 MBq/nmol. In vitro 47Sc-cm10 showed folate receptor–specific binding and uptake into KB tumor cells. In vivo SPECT/CT images allowed the visualization of accumulated radioactivity in KB tumors and in the kidneys. The therapy study showed a significantly delayed tumor growth in mice, which received 47Sc-cm10 (10 MBq, 10 Gy) resulting in a more than 50% increase in survival time, compared with untreated control mice. Conclusion: With this study, we demonstrated the suitability of using 47Sc for therapeutic purposes. On the basis of our recent results obtained with 44Sc-folate, the present work confirms the applicability of 44Sc/47Sc as an excellent matched pair of nuclides for PET imaging and radionuclide therapy.
47Sc nuclear reaction at the high-flux reactor at the Institut Laue-Langevin. Separation of the 47Sc from the target material was performed by a semi-automated process using extraction chromatography and cation exchange chromatography. 47Sc-labeled cm10 was tested on folate receptor–positive KB tumor cells in vitro. Biodistribution and SPECT imaging experiments were performed in KB tumor–bearing mice. Radionuclide therapy was conducted with two groups of mice, which received either 47Sc-cm10 (10 MBq) or only saline. Tumor growth and survival time were compared between the two groups of mice. Results: Irradiation of 46Ca resulted in approximately 1.8 GBq of 47Ca, which subsequently decayed to 47Sc. Separation of 47Sc from 47Ca was obtained with 80% yield in only 10 min. The 47Sc was then available in a small volume (∼500 μL) of an ammonium acetate/HCl (pH 4.5) solution suitable for direct radiolabeling. 47Sc-cm10 was prepared with a radiochemical yield of more than 96% at a specific activity of up to 13 MBq/nmol. In vitro 47Sc-cm10 showed folate receptor–specific binding and uptake into KB tumor cells. In vivo SPECT/CT images allowed the visualization of accumulated radioactivity in KB tumors and in the kidneys. The therapy study showed a significantly delayed tumor growth in mice, which received 47Sc-cm10 (10 MBq, 10 Gy) resulting in a more than 50% increase in survival time, compared with untreated control mice. Conclusion: With this study, we demonstrated the suitability of using 47Sc for therapeutic purposes. On the basis of our recent results obtained with 44Sc-folate, the present work confirms the applicability of 44Sc/47Sc as an excellent matched pair of nuclides for PET imaging and radionuclide therapy.
The concept of radiotheragnostic applications is based on the employment of nuclides of the same element allowing the use of chemically identical radiopharmaceuticals for both diagnosis and therapy (1). Several matched pairs of radionuclides have been proposed for this purpose (Table 1; Fig. 1). The best established example is iodine, which has been used for several decades in SPECT (123I) and PET (124I) imaging, as well as for β− radio-nuclide therapy (131I) (2). In terms of radiometals, yttrium and copper are elements that comprise radionuclides useful for clinical PET imaging (86Y and 61/62/64Cu) and for targeted β− radionuclide tumor therapy (90Y/64/67Cu) (3–6). Terbium nuclides have been proposed recently for PET (152Tb) and SPECT imaging (155Tb) as well as for α (149Tb) and β− radionuclide therapy (161Tb); however, these nuclides have not been made available for clinical application yet (7–9).
Nuclear Data of Theragnostic Isotopes for Therapy, PET, and SPECT Imaging
Biodistribution of 47Sc-cm10 in KB Tumor–Bearing Nude Mice
Theragnostic principle: matched pairs of radionuclides for PET or SPECT imaging and for therapeutic application in nuclear medicine. Radionuclides are designated according to Karlsruhe’s Chart of Nuclides.
In terms of radiotheragnostics, scandium, a trivalent rare earth metal, is of particular interest (Fig. 1) (1). 44Sc has been recently proposed and investigated for PET imaging, because it decays by the emission of positrons (average energy [Eβ− average], 632 keV; intensity, 94.3%), with a half-life (T1/2) of 3.97 h (10). These properties would potentially allow the distribution of 44Sc-labeled radiopharmaceuticals to hospitals without cyclotron and radiopharmaceutical laboratories available.
47Sc was proposed as a potential therapeutic match to the PET nuclide 44Sc. It is a low-energy β− emitter with decay characteristics (T1/2, 3.35 d; Eβ−av,162 keV) that are potentially useful for radionuclide tumor therapy similar to the clinically established 177Lu (T1/2, 6.65 d; Eβ−av, 134 keV; Eγ, 113, 208 keV). The most obvious difference between 47Sc and 177Lu is the significantly shorter half-life of 47Sc. This characteristic would be particularly favorable for the combination of 47Sc with small-molecular-weight and peptide-based targeting ligands with a relatively fast blood clearance. Moreover, 47Sc has been proposed for radioimmunotherapy (11). Under the assumption that it forms stable complexes with open-chained chelators (e.g., octadentate diethylene triamine pentaacetic acid) as recently proposed (12), radiolabeling of antibodies would become accessible at room temperature. Similar to 177Lu, the decay of 47Sc is also accompanied by the emission of γ-rays of an ideal energy (Eγ, 159 keV; intensity, 68.3%) for SPECT imaging. For all of these reasons, 47Sc is a highly promising new candidate of a radionuclide for potential application in therapeutic nuclear medicine.
The goal of the present study was to investigate 47Sc as a therapeutic pendant to the recently tested PET nuclide 44Sc (13) using the same targeting principle. The DOTA-folate conjugate (cm10) used in this study is composed of the same functionalities as was the case for the previously evaluated folate conjugate cm09 (14). Folic acid binds selectively to the folate receptor (FR), which is expressed on a variety of tumor types, including cancer of the ovaries and of the lungs (15,16). The DOTA-chelator can be used for stable coordination of Sc as previously demonstrated (13,17). Finally, there is a small-molecular-weight albumin-binding entity (18) integrated in the folate conjugate cm10. This additional functionality was shown to enhance the blood circulation time of folate conjugates and, as a consequence, to improve the tissue distribution profile (13). In the present study, 47Sc-cm10 was evaluated in vitro using FR-positive KB tumor cells and in vivo by the performance of biodistribution and SPECT/CT studies. Radionuclide therapy was conducted with two groups of KB tumor–bearing mice, which received either 47Sc-cm10 or saline only. The average tumor growth and survival time were compared between treated animals and untreated controls.
MATERIALS AND METHODS
Production of 47Sc
47Ca was produced via the 46Ca(n,γ)47Ca nuclear reaction by irradiation of a 46Ca target (dried nitrate, 1 mg of metal mass, 31.7% enrichment of 46Ca) for 3.94 d in a thermal neutron flux of 1.5 × 1015 cm−2s−1 at the high-flux reactor of Institut Laue-Langevin in Grenoble, France. 47Ca decays to 47Sc, with a half-life of 4.54 d, by the emission of β− particles and γ-rays. This allowed repeated chemical separation of the daughter nuclide 47Sc in a pseudogenerator-like system. For the separation of 47Sc(III) from Ca(II) (47Ca and stable calcium isotopes), a previously developed semiautomated separation system was used (13). In brief, the irradiated target was dissolved in HCl (3 M, prepared from 30% HCl, Suprapur [Merck KGaA]) and loaded onto a column containing N,N,N’,N’-tetra-n-octyldiglycolamide (DGA, 50–100 μm [Triskem International]) extraction resin. The adsorbed 47Sc(III) was eluted from the DGA resin with HCl (0.1 M, ∼3 mL). The acidic 47Sc(III) eluate (0.1 M HCl) was loaded onto a second column containing a cation exchange resin in water (DOWEX 50, hydrogen form, 200–400 mesh [Fluka Analytic]). 47Sc(III) was eluted with a mixture of CH3COONH4 and HCl (0.75 and 0.2 M, respectively; ∼500 μL, pH 4.5; CH3COONH4: Trace SELECT, ≥ 99.9999%, Fluka Analytic; HCl 30%: Suprapur, Merck KGaA).
Radiosynthesis
The organic synthesis of the albumin-binding DOTA-folate (cm10) is reported in the supplemental data (available at http://jnm.snmjournals.org). For the preparation of 47Sc-cm10, a stock solution of cm10 (10 μL, 10−3 M) was added to the solution of 47Sc in CH3COONH4/HCl (∼250 μL; 130 MBq, pH ∼4.5) and incubated at 95°C for 10 min. Sodium diethylene triamine pentaacetic acid (5 μL, 5 mM, pH 5) was added to the reaction mixture for the complexation of potential traces of free 47Sc(III). Quality control was performed by high-performance liquid chromatography. For preclinical application, variable amounts of cm10 were added to obtain the required specific activity. For in vivo application, the labeling mixture containing 47Sc-cm10 was diluted with 3 parts of an equivalent volume of MilliQ water to reduce the osmolarity (365 mOsm). In vitro stability and cell experiments are reported in the supplemental data.
Biodistribution Studies
In vivo experiments were approved by the local veterinarian department and conducted in accordance with the Swiss law of animal protection. Four- to 5-wk-old female athymic nude mice (CD-1 Foxn-1/nu) were purchased from Charles River Laboratories. The animals were fed a folate-deficient rodent diet (ssniff Spezialdiät10 GmbH) starting 5 d before KB tumor cell (5 × 106 cells in 100 μL of phosphate-buffered saline) inoculation into the subcutis of each shoulder. Biodistribution studies were performed in triplicate approximately 14 d after cell inoculation. 47Sc-cm10 (2 MBq, 1 nmol/mouse) was injected in a volume of 100 μL into a lateral tail vein. The animals were sacrificed at pre-determined time points after administration of 47Sc-cm10. Selected tissues and organs were collected, weighed, and counted for radioactivity using a γ-counter. The results were listed as a percentage of the injected activity per gram of tissue mass (%IA/g), using counts of a defined volume of the original injection solution counted at the same time. Dosimetric calculations were performed on the basis of these data (supplemental data).
SPECT Studies
SPECT/CT studies were performed with a 4-head multiplexing multipinhole camera (NanoSPECT/CT; Mediso Medical Imaging Systems). Each head was outfitted with a tungsten-based aperture of nine 1.4-mm-diameter pinholes and a thickness of 10 mm. SPECT/CT images were acquired by use of Nucline software (version 1.02; Bioscan). CT scans were obtained with the integrated CT using a tube voltage of 55 kVp and an exposure time of 1.0 s per view. After the acquisitions, SPECT data were reconstructed iteratively with HiSPECT software (version 1.4.3049; Scivis GmbH) using the γ-energy of 159 keV ± 10% of 47Sc. The real-time CT reconstruction used a cone-beam filtered backprojection. SPECT and CT data were automatically co-registered, because both modalities shared the same axis of rotation. The fused datasets were analyzed with the InVivoScope postprocessing software (version 1.44; Bioscan Inc.).
In vivo SPECT/CT imaging studies were performed with a nude mouse approximately 14 d after KB tumor cell inoculation. 47Sc-cm10 (∼13 MBq, 1 nmol/mouse) was intravenously injected. For the in vivo scan, the mouse was anesthetized by inhalation of an isoflurane–oxygen mixture. The scans were obtained with a time-per-view of 100–350 s, resulting in a scan time of about 1 h (48 h after injection in vivo) and 4.5 h (96 h after injection post-mortem), respectively. All SPECT scans were preceded by a CT scan.
Preclinical Therapy Study Using 47Sc-cm10
KB cells (4.5 × 106 cells in 100 μL of phosphate-buffered saline) were subcutaneously injected 4 d before the start of therapy at day 0. Two groups (groups A and B) consisting of 6 mice each were injected with only saline (group A, control) or with 47Sc-cm10 (group B, 10 MBq, 1 nmol) at day 0 when the average KB tumor volume reached 53 ± 24 mm3 in the mice of group A and 61 ± 20 mm3 in the mice of group B. The mice were weighed 3 times a week over a period of about 7 wk. The relative body weight (RBW) was defined as [BWx/BW0], where BWx is the body weight in grams at a given time x and BW0 the body weight at day 0. The tumor volume was determined by measuring 2 dimensions with a digital caliper and calculated according to the equation [0.5 × (L × W2)], where L is the longest axis and W the axis perpendicular to L in millimeters (19). The relative tumor volume (RTV) was defined as [TVx/TV0], where TVx is the tumor volume in mm3 at a given time x and TV0 the tumor volume at day 0. The efficacy of 47Sc-cm10–based therapy was expressed as the percentage tumor growth inhibition (TGI), calculated using the equation [100 − (T/C × 100)], where T is the mean RTV of the treated mice and C is the mean RTV in the control group at the time of euthanasia of the first mouse of the control group (20). Tumor growth delay (TGDx) was calculated as the time required for the tumor volume to increase x-fold over the initial volume at the day 0. The tumor growth delay index [TGDIx = TGDx(T)/TGDx(C)] was calculated as the TGDx ratio of treated mice (T) over control mice (C) for a 5-fold (x = 5, TGD5) and 10-fold (x = 10, TGD10) increase of the initial tumor volume.
Endpoint criteria were defined as body weight loss of more than 15% of the initial body weight (at day 0), KB tumor volume more than 1,000 mm3, ulceration or bleeding of the tumor xenograft, or abnormal behavior indicating pain or unease of the animal. Mice were removed from the study and euthanized on reaching one of the predefined endpoint criteria. To calculate significance of the survival time and tumor growth delay, a t test (Excel software; Microsoft) was used. All analyses were 2-tailed and considered as type 3 (2-sample unequal variance). A P value of less than 0.05 was considered statistically significant.
RESULTS
Production of 47Sc
Irradiation of the 46Ca target (1 mg) for 3.94 d at the high-flux reactor of Institut Laue-Langevin resulted in the production of approximately 1.8 GBq of 47Ca and approximately 0.6 GBq of 47Sc at the end of irradiation. After shipment of the target to the Paul Scherrer Institute, γ-ray spectrometry was performed with an aliquot of the dissolved target solution. Apart from 47Ca (Eγ, 489.2, 807.9, and 1,297.1 keV) and its decay product 47Sc (Eγ, 159.4 keV), no radionuclidic impurities were detectable (supplemental data).
Separation of 47Sc
At the time of the first separation of 47Sc(III) from carrier-added 47Ca(II) (5.8 d after end of irradiation), the activity of 47Sc was approximately 900 MBq. The separation of 47Sc was performed using extraction chromatography and cation exchange chromatography within approximately 10 min, as previously reported (Fig. 2) (13). A DGA column served for adsorption of 47Sc from 3 M HCl whereas the 47Ca remained in solution and was eluted (Fig. 2A). Then, the 47Sc(III) was eluted with 0.1 M HCl (Fig. 2B). For subsequent concentration of the 47Sc solution, a DOWEX 50–based cation exchange chromatography column was used. 47Sc (∼740 MBq in ∼500 μL; first separation) was formulated at a radioactivity concentration of up to approximately 1.5 GBq/mL in a solution (0.75 M NH4Ac/0.2 M HCl, pH ∼4.5) that was suitable for a direct radiolabeling process (Fig. 2C). A second separation of approximately 240 MBq of 47Sc was performed 3.9 d later, after renewed generation of 47Sc (∼300 MBq). The overall yield of 47Sc after separation was approximately 80%, and the amount of 47Ca in the collected 47Sc fraction was less than 1% (supplemental data).
Separation process of 47Sc from calcium in 3 steps. (A) Dissolved target is loaded onto column I where 47Sc is adsorbed and calcium is eluted. (B) 47Sc is eluted and directly loaded onto column II where it is adsorbed. (C) Elution of 47Sc from column II.
Preparation and In Vitro Evaluation of 47Sc-cm10
The preparation of 47Sc-cm10 was performed as previously reported (Fig. 3) (13). Quality control performed by high-performance liquid chromatography showed the product peak of 47Sc-cm10, with a retention time of 19.3 min. The radiochemical yield was more than 96% at a specific activity of up to 13 MBq/nmol, representing a 47Sc-to-ligand molar ratio of 1:110. 47Sc-cm10 was stable in phosphate-buffered saline with only minimal release of 47Sc(III) (<3%) within the first 24 h. Uptake and internalization of 47Sc-cm10 into KB tumor cells was increasing over time. FR-specific binding was proven by the fact that uptake of 47Sc-cm10 was reduced to less than 1% in cell samples, which were co-incubated with excess folic acid to block the receptors (Fig. 4).
Chemical structure of 47Sc-labeled DOTA-folate conjugate (cm10) with speculative coordination sphere of scandium radionuclide.
Time-dependent uptake and internalization of 47Sc-cm10 in KB cells. Values are indicated as percentage of total added radioactivity per 0.15 mg of protein. Co-incubation with excess folic acid resulted in blocked uptake (<1%) of 47Sc-cm10.
Biodistribution Studies
Biodistribution studies with 47Sc-cm10 showed a high accumulation of radioactivity in the tumor tissue, with a maximum value of 18.0 ± 2.2 %IA/g at 4 h after injection and an excellent retention over time (11.7 ± 1.5 %IA/g at 72 h after injection) (Table 2). Radioactivity measured in the blood was decreasing rapidly from 5.8 ± 1.1 %IA/g at 1 h after injection to background levels after 1 d (0.4 ± 0.1 %IA/g). Significant accumulation of radioactivity was also found in the kidneys (28.8 ± 3.9 %IA/g; 24 h after injection) and in the salivary glands (3.8 ± 0.7 %IA/g; 24 h after injection) where the FR is expressed. In the liver, the uptake was relatively high shortly after the injection of 47Sc-cm10 (4.4 ± 0.4 %IA/g; 1 h after injection) but decreased constantly over time to 1.6 ± 0.7 %IA/g at 96 h after injection. In all other tissue and organs such as lung, spleen, stomach, intestines, muscle, and bone, retention of radioactivity was low and decreased further over time.
For tumor xenografts and kidneys, the absorbed dose was calculated as approximately 1.0 and 2.0 Gy/MBq, respectively, resulting in an absorbed tumor dose of approximately 10 Gy and a kidney dose of 20 Gy on a single injection of 10 MBq of 47Sc-cm10.
In Vivo SPECT Imaging Studies Using 47Sc-cm10
SPECT/CT studies were performed with a KB tumor–bearing mouse 48 h after injection of 47Sc-cm10 (Fig. 5), enabling excellent visualization of the tumors, the sites of highest accumulation of radioactivity. Besides, uptake of radioactivity was seen only in the kidneys. This is always observed after injection of folate-based radioconjugates because of their specific binding to FRs expressed in the proximal tubule cells. However, in the liver, lung, spleen, and intestinal tract, radioactivity was not retained.
SPECT/CT image of KB tumor–bearing mouse 48 h after injection of approximately 13 MBq of 47Sc-cm10. Ki = kidney; Tu = tumor.
Preclinical Therapy Study Using 47Sc-cm10
In the mice of group A, which received only saline, the KB tumors were growing constantly over time, whereas in the 47Sc-cm10–treated mice of group B the tumor growth was clearly delayed (Fig. 6A). At day 21 of the study, the first control mouse (group A) had to be euthanized because of an oversized tumor. At that time point, the calculated tumor growth index revealed a value of 73% (Table 3). The tumor growth delay inhibition of treated mice calculated for an RTV of 5 (TGDI5) was 2.0 ± 0.6, indicating a 2-fold increased time for tumors to reach the same volume as the control animals. To reach an RTV of 10, the required time had increased 1.5-fold in treated animals, compared with untreated control animals, reflected by a TGDI10 of 1.5 ± 0.3 (Table 3).
RTV (A), RBW (B), and survival (C) of mice in preclinical therapy study. Mice of group A received saline, and mice of group B received 10 MBq of 47Sc-cm10. Average survival time was 25 d (group A) and 38.5 d (group B).
Results of Therapy Study with 47Sc-cm10
After injection of 47Sc-cm10, the mice experienced slight body weight loss (Fig. 6B). Throughout the investigation, the average RBW of treated mice (group A) was somewhat lower than the RBW of untreated control mice, but extensive body weight loss (>15%) was not observed. The average survival time was 25 d for control mice and 38.5 d for treated mice, which meant an additional survival time of 54% in the case of 47Sc-cm10 therapy (Fig. 6C).
DISCUSSION
In the past, 47Sc has been proposed as a new radionuclide for application in therapeutic nuclear medicine (11,21). Herein, we reported on the first, to our knowledge, preclinical in vivo study performed with a 47Sc-labeled small-molecular-weight biomolecule for tumor targeting. In this respect, a novel DOTA-folate conjugate (cm10) was used. In vitro 47Sc-cm10 showed results comparable to the previously investigated 177Lu-cm09 (14). In vivo, 47Sc-cm10 was assessed in biodistribution studies over 7 d using KB tumor–bearing mice. High uptake of 47Sc-cm10 was found in tumor xenografts and in the kidneys, because both of these tissues express the FR substantially. In the blood and in non-targeted organs and tissues, retention of radioactivity decreased over time, reaching background levels after about 24 h. These data were comparable to, but not completely the same as, those previously obtained with 177Lu-cm09 (14). Potential reasons for certain discrepancies could be the fact that the experiments with 177Lu-cm09 and 47Sc-cm10 were not performed in parallel (interexperimental variability) and that these radioconjugates differed not only with regard to the used radionuclide (47Sc vs. 177Lu) but also with regard to the chemical structure of the DOTA-folate conjugate (cm10 vs. cm09). SPECT/CT imaging experiments obtained after injection of 47Sc-cm10 in KB tumor–bearing mice confirmed the post-mortem tissue distribution data. The images showed also an excellent analogy to the PET scans obtained with mice after injection of 44Sc-cm10 (supplemental data). Additional SPECT studies were performed with Derenzo phantoms to compare 47Sc with 177Lu. The images showed an equally high resolution for both nuclides, confirming the suitability of using 47Sc for SPECT imaging, which would be important for pre-therapeutic dosimetry in patients (supplemental data).
The most crucial part for the assessment of 47Sc was the performance of a therapy experiment, which was conducted with the standard KB tumor mouse model according to a protocol previously used for 177Lu-cm09 and 161Tb-cm09 (9). The estimation of the absorbed tumor dose after injection of 10 MBq of 47Sc-cm10 revealed a value of about 10 Gy. The significant tumor growth delay observed in treated mice resulted in additional survival time (+54%), compared with untreated controls. Despite the much lower tumor dose, the data suggest a comparable antitumor efficacy of 47Sc-cm10 with the previously evaluated 177Lu-cm09 (∼24 Gy) and 161Tb-cm09 (∼33 Gy) (9) at the same quantity of injected activity (10 MBq/mouse) and in the same tumor mouse model. Because of the limited availability of 47Sc, kidney toxicity studies have not been performed yet. However, it is likely that renal damage, which has been observed with other therapeutic folate radioconjugates, would be absent at such low dose levels of only 20 Gy to the kidneys. This assumption is based on the commonly used kidney dose limit of approximately 23 Gy, which was found to be safe during external radiation therapy (22). Should the findings of this study be confirmed in further in vivo studies, 47Sc may be of considerable interest for a clinical application of targeted radionuclide tumor therapy.
In the near future, 47Sc should be made available at sufficient quantities to allow further and more extended preclinical therapy studies. In the present study, the production of 47Sc was accomplished via the 46Ca(n,γ)47Ca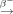 47Sc nuclear reaction. Enriched 46Ca was irradiated at a high-flux reactor to produce 47Ca, which, in turn, decays to 47Sc. The application of such a radionuclide pseudogenerator system provides an opportunity to separate the daughter nuclide 47Sc from the mother nuclide 47Ca several times. However, a significant drawback of this production route is the high price of enriched 46Ca as a result of its extremely low abundance of only 0.004% in natural calcium. Alternative routes for the production of 47Sc have been reported in the literature (23–26). Among these, the most feasible appears to be the irradiation of 47Ti targets with fast neutrons to induce the 47Ti(n,p)47Sc nuclear reaction (25,27,28). In this respect, more investigations will be necessary to evaluate the production at a larger scale and optimize the isolation conditions of 47Sc. If this will be successful, application of 47Sc in preclinical and most likely also clinical studies will be approachable in the future.
47Sc nuclear reaction. Enriched 46Ca was irradiated at a high-flux reactor to produce 47Ca, which, in turn, decays to 47Sc. The application of such a radionuclide pseudogenerator system provides an opportunity to separate the daughter nuclide 47Sc from the mother nuclide 47Ca several times. However, a significant drawback of this production route is the high price of enriched 46Ca as a result of its extremely low abundance of only 0.004% in natural calcium. Alternative routes for the production of 47Sc have been reported in the literature (23–26). Among these, the most feasible appears to be the irradiation of 47Ti targets with fast neutrons to induce the 47Ti(n,p)47Sc nuclear reaction (25,27,28). In this respect, more investigations will be necessary to evaluate the production at a larger scale and optimize the isolation conditions of 47Sc. If this will be successful, application of 47Sc in preclinical and most likely also clinical studies will be approachable in the future.
CONCLUSION
In this study, the promising potential of 47Sc was demonstrated for the first time in combination with a small-molecular-weight targeting agent in a preclinical setting. Excellent features of 47Sc for application in therapeutic nuclear medicine have been confirmed. 47Sc is in particular attractive as part of the theragnostic principle together with 44Sc, which may be used for pre-therapeutic imaging as well as therapy planning and monitoring. In view of these promising future prospects for 47Sc, further preclinical experiments, using larger cohorts of animals and variable targeting agents, will be necessary. Moreover, comparison of 47Sc with the clinically established β− emitter 177Lu will be crucial to draw final conclusions about the suitability of 47Sc for clinical application.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. The project was financially supported by the Swiss Cancer League (KLS-02762-02-2011). No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Dr. Konstantin Zhernosekov for initiating the study and Martin Hungerbühler for technical assistance.
Footnotes
Published online Jul. 17, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication April 14, 2014.
- Accepted for publication June 9, 2014.













