Abstract
The 11C-labeled tracer meta-hydroxyephedrine (11C-HED) is a noradrenaline analog that was developed to visualize the sympathetic nervous system with PET. Initial clinical studies show a rapid uptake of 11C-HED in localized tumors of this system. Whole-body imaging with 11C-HED PET is now possible as PET/CT scanners allow a rather short examination time. The aim of this study was to evaluate the feasibility of whole-body 11C-HED PET/CT for examination of tumors of the sympathetic nervous system and to directly compare the results with 123I-labeled meta-iodobenzylguanidine (123I-MIBG) scintigraphy, including SPECT/CT. Methods: In 19 consecutive patients, 9 mo to 68 y old (median, 32 y), 24 whole-body 11C-HED PET/CT (low-dose CT) examinations were performed. Scans were compared with attenuation-corrected 123I-MIBG SPECT/CT scans (24-h scan, low-dose CT). The intensity of tracer accumulation above background was visually analyzed in both scans, PET and SPECT, using a 4-value scale. In 11C-HED PET, mean and maximum standardized uptake values were determined for all lesions. Results: In 14 patients with 19 pairs of examinations, the following tumors were confirmed histologically: 6 neuroblastomas, 5 pheochromocytomas, 1 ganglioneuroblastoma, and 2 paragangliomas. In 5 patients, each having 1 pair of examinations, clinical follow-up and/or histologic examination did not reveal any tumor deriving from the sympathetic nervous system. 11C-HED PET/CT detected 80 of 81 totally depicted tumor lesions (sensitivity, 0.99; soft tissue, 61; bone, 19). 123I-MIBG SPECT/CT detected 75 of 81 lesions (sensitivity, 0.93; soft tissue, 56; bone, 19). With both methods, there were no false-positive lesions. The tumor-to-background contrast of 11C-HED uptake was higher in comparison with 123I-MIBG uptake in 26 lesions (0.32; soft tissue, 18; bone, 8), equal in 39 lesions (0.48; soft tissue, 30; bone, 9), and lower than 123I-MIBG uptake in 16 lesions (0.20; soft tissue, 14; bone, 2). Conclusion: Whole-body imaging using 11C-HED PET/CT is feasible in the clinical setting of patients with tumors of the sympathetic nervous system. 11C-HED PET/CT detected more tumor lesions than 123I-MIBG SPECT/CT. However, tumor-to-background contrast of 11C-HED in lesions can be higher, equal, or lower compared with 123I-MIBG.
Neuroblastomas, pheochromocytomas, ganglioneuroblastomas, and paragangliomas are tumors deriving from the sympathetic nervous system. These tumors originate from the adrenal gland and sympathetic ganglions anywhere from the neck to the pelvis. In the case of malignant tumors, metastases can be found in soft tissue, bone, and bone marrow. Because of their neuroendocrine origin these tumors are able to take up catecholamines and related substances. The diagnosis of these tumors is established biochemically by measuring the level of urinary and plasma catecholamines and their metabolites. Today there are several morphologic and functional imaging methods available that predict tumor localization and tumor extent and give anatomic information (1). CT and MRI are the morphologic imaging modalities of choice in localizing these tumors (2). Both provide excellent anatomic details and sensitivity is very high. Both are lacking in specificity as difficulties may occur in distinguishing between tumors deriving from the sympathetic nervous system and other tumor entities (1). The major advantages of radionuclide imaging are high sensitivity, very high specificity, and the routinely performed whole-body scanning. Furthermore, in follow-up examinations, functional imaging is not affected by postoperative artifacts such as scar tissue or metallic clips.
The catecholamine analog 123I-meta-iodobenzylguanidine (123I-MIBG) is the radiotracer most widely used to image tumors of the sympathetic nervous system. With regard to its physical characteristics and kinetics, 123I-MIBG is less than ideal for imaging. In contrast, 11C-labeled meta-hydroxyephedrine (11C-HED) is a catecholamine analog that has been developed specifically for imaging the sympathetic nervous system using PET (3). 11C-HED uptake reflects catecholamine transport, storage, as well as catecholamine recycling (4). Preliminary kinetic studies suggested a rapid accumulation and high retention of 11C-HED in localized neuroblastomas and pheochromocytomas (5–7). However, to our knowledge, whole-body 11C-HED PET for the detection of extraadrenal tumors and metastases has not yet been performed in a larger patient cohort (8,9). Fast PET/CT hybrid scanners enable attenuation-corrected, high-quality whole-body images of radionuclides with a short half-life. The purpose of this study was to evaluate the feasibility of whole-body 11C-HED PET/CT with low-dose CT in a clinical setting in all age groups and to directly compare the results with 123I-MIBG single-photon emission tomography combined with low-dose CT (SPECT/CT).
MATERIALS AND METHODS
Patients
Within a 20-mo period of time, all patients with known or suspected tumors of the sympathetic nervous system who were referred for 123I-MIBG scintigraphy were included in this investigation. Twenty-four pairs of examinations (123I-MIBG scintigraphy and 11C-HED PET/CT) were performed on 19 patients, age 9 mo to 68 y old (median, 32 y). The time interval between the 11C-HED PET/CT and 123I-MIBG scintigraphy was <4 wk for all examinations (median, 6 d; range, 0–25 d), and within this interval no therapy or intervention was performed. In 14 pairs of examinations, chemotherapy, 131I-MIBG therapy, and/or resection of the tumor preceded the scans and the patients had recurrent or persistent disease. In 10 pairs of examinations, no therapy was performed previously. None of the patients was on medication known to interfere with the cellular uptake of catecholamines. Whenever necessary, young children were sedated or anesthetized for imaging by a pediatrician or anesthesiologist.
This investigation was approved by the local ethics committee for adults (all tumor types) and for children (neuroblastoma only). This study had to include children, as neuroblastomas do not occur in adult patients. All patients or parents (in the case of pediatric patients) gave written informed consent.
11C-HED PET/CT
The synthesis of 11C-HED has been previously described in detail (4). Patients received an intravenous injection of 320 MBq 11C-HED (median; range, 125–716 MBq). In children, the injected activity was corrected for body weight starting with 370 MBq for a 70-kg individual and scaling down according to recommendations of the pediatric task group of the European Association of Nuclear Medicine (EANM). PET/CT studies were performed using a hybrid scanner (Biograph Sensation 16; Siemens Medical Solutions). Whole-body unenhanced, low-dose multidetector-row CT (MDCT) for attenuation correction and anatomic land marking was started immediately after the injection with the patients holding their breath in mild expiration (detector configuration, 16 × 0.75 mm; gantry rotation time, 420 ms; tube voltage, 120 kV; effective tube current, 13–20 mAs, online tube current modulation [Care Dose; Siemens Medical Solutions]; table feed, 30 mm/rotation; field of view, at least from the base of the skull to the middle of the thigh, CT parameters were adapted to body weight or axial diameter in children). Whole-body PET acquisition was started 5 min after the injection (field of view from the base of the skull to the middle of the thigh, 4-min emission scan per bed position). Data from low-dose MDCT were reconstructed in an overlapping manner at 2-mm slice thickness with a 1-mm reconstruction increment, using the standard soft-tissue reconstruction kernel B30, the lung reconstruction kernel B50, and the bone reconstruction kernel B60. Attenuation-corrected PET images were reconstructed iteratively. For CT data analysis, the window width were set to 350 Hounsfield units (HU) and the window center was set to 50 HU (soft tissue), 1,700 HU/−500 HU (lung parenchyma), and 320 HU/800 HU (bone structures).
123I-MIBG Scintigraphy and SPECT/CT
123I-MIBG was injected intravenously at least 30 min after blocking the thyroid gland by orally given 300 mg sodium perchlorate. In children, activity was given weight adapted according to recommendations of the pediatric task group of the EANM using 370 MBq for 70-kg body weight. Whole-body scintigraphy in anterior and posterior views, lateral views of the head, and single SPECT of the primary tumor region were performed 4 and 24 h after injection. Planar scintigraphy was acquired with a dual-head whole-body γ-camera (Bodyscan, Siemens; or Hawkeye, GE Healthcare) with a scanning velocity of 5 cm/min. SPECT was acquired with a dual-head γ-camera (Hawkeye), equipped with a medium-energy collimator. Thirty-two projections, 40 s each, were acquired over a 180° rotation in a 64 × 64 matrix. Low-dose CT was performed with the 24-h postinjection acquisition for attenuation correction and anatomic orientation (10). SPECT images without (4 h after injection) and with attenuation correction (24 h after injection) were reconstructed iteratively.
Data Analysis and Interpretation
11C-HED PET and low-dose MDCT were transferred to a Leonardo workstation (VA 70; Siemens Medical Solutions) for further assessment. 11C-HED PET and 123I-MIBG scintigraphy were analyzed independently, each by 2 experienced nuclear medicine physicians who knew the patients' data and clinical symptoms but were unaware of other imaging results. In case of disagreement between both readers, consensus was obtained. Any focal tracer uptake in the adrenal glands or extraadrenal regions that exceeded the normal regional tracer uptake was considered a pathologic lesion. Pathologic lesions were categorized semiquantitatively using a 4-value scale (1 = faintly, 2 = moderately, and 3 = highly increased tracer uptake above background). A site was classified as 0 (no increased tracer uptake) in case a tumor manifestation was not visible with the assessed method. Analysis was performed on a computer monitor in all 3 planes of the 11C-HED PET and the 24-h postinjection 123I-MIBG SPECT. Furthermore, any pathologic lesion was localized by image fusion with the low-dose CT. Low-dose MDCT scans of the PET/CT were read in the soft-tissue, bone, and pulmonary window by 2 experienced radiologists. Low-dose MDCT was used to determine the size of the lesions. Additionally, in the 11C-HED PET, mean and maximum standardized uptake values (SUVmean, SUVmax) were assessed for all lesions and for normal organs. Tumor sites that were not within the field-of-view on 24-h postinjection 123I-MIBG SPECT were excluded from the semiquantitative analyses and the direct comparison of both tomographic methods, 11C-HED PET/CT and 123I-MIBG SPECT/CT.
Morphologic Imaging
All patients underwent additional morphologic imaging using contrast-enhanced MDCT, MRI, or ultrasound during their clinical work-up.
Gold Standard
At least one tumor site was confirmed histologically in all tumor patients studied. In patients with multiple tumor manifestation, not all sites were verified histologically because of the advanced stage of disease and the systemic therapy as the first therapeutic choice. To clarify discrepant results between 11C-HED PET and 123I-MIBG SPECT, low-dose CT, the other morphologic imaging methods, and the clinical and imaging follow-up were used. If either PET or SPECT was negative in a region with a clearly visible lesion in morphologic imaging, the negative finding was considered false-negative. In case of clearly positive PET or SPECT but negative morphologic imaging, clinical and imaging follow-up were considered.
Statistics
Normal distribution was tested by the Kolmogorov–Smirnov test. Sensitivities were calculated on a lesion-by-lesion and examination-by-examination basis. The 95% confidence intervals (95% CI) are given for these parameters.
RESULTS
Fourteen patients were included in this study after histologic confirmation of the following tumors: 6 neuroblastomas, 5 pheochromocytomas, 1 ganglioneuroblastoma, and 2 paragangliomas (Table 1). These 14 patients with a total of 19 pairs of examinations constitute the study group (10 patients with 1 pair, 3 patients with 2 pairs, and 1 patient with 3 pairs of examinations). For 123I-MIBG scintigraphy, a total of 6 sedations were performed (3 neuroblastoma patients, 4 and 24 h after injection). In the same 3 patients, two 11C-HED PET/CT examinations were performed under sedation and 1 was performed under general anesthesia (radiotherapy planning CT in the same session).
Characterization of Tumor Patients
In 5 patients referred for the evaluation of suspected pheochromocytoma and each having 1 pair of examinations, 11C-HED PET/CT, 123I-MIBG scintigraphy, and SPECT/CT, clinical and imaging follow-up and/or histologic examination did not reveal any tumor deriving from the sympathetic nervous system. The 11C-HED PET/CT examinations of these patients were analyzed to describe the normal whole-body 11C-HED distribution.
Normal Whole-Body 11C-HED Distribution
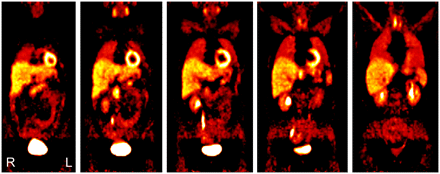
With exception of the urogenital system and the thyroid gland, all 5 persons in whom no tumor of the sympathetic nervous system could be verified showed a uniform distribution pattern of 11C-HED in the body (Fig. 1; Table 2). Very low tracer accumulation was shown in soft tissue, muscle, gut, lungs, and bones. Homogeneous moderate tracer accumulation was shown in salivary glands, spleen, and adrenal glands. Homogeneous high uptake was demonstrated in myocardium, liver, and pancreas. Variable high 11C-HED accumulation was demonstrated in renal parenchyma, renal pelvis, ureter, urinary bladder, and thyroid gland.
A 43-y-old woman with normal whole-body distribution on 11C-HED PET scan (several levels of coronal slices) with high 11C-HED accumulation in renal pelvis, ureter, urinary bladder, myocardium, liver, thyroid, and pancreas and moderate tracer uptake in salivary glands and spleen. Adrenal glands demonstrate only very low 11C-HED uptake and can only be differentiated from surrounding tissue by using PET/CT fusion image.
Normal Distribution of 11C-HED (n = 5)
Tumor Patients
Lesions Within Field of View of 123I-MIBG SPECT/CT.
Using 11C-HED PET/CT, 80 of 81 totally detected lesions showed increased tracer uptake, 19 osseous lesions and 61 soft-tissue lesions (Tables 3 and 4). Despite the massive physiologic 11C-HED accumulation in the renal collecting system and the high pancreatic 11C-HED uptake, lesions next to these organs could be unequivocally differentiated in all cases by means of 11C-HED PET/CT fusion images. 123I-MIBG SPECT/CT 24 h after injection demonstrated a total of 75 of 81 lesions, 19 osseous lesions and 56 soft-tissue lesions. There were no additional lesions on the 4-h postinjection 123I-MIBG SPECT images. There was only 1 additional lesion on 123I-MIBG SPECT not showing up on the PET scan by an increased 11C-HED accumulation. This lesion was a large local relapse of a neuroblastoma and was visible on the low-dose CT component of the PET/CT (4.2 × 4.6 × 5.3 cm). This positive 123I-MIBG finding had a clear influence on further therapy. Six lesions in 3 patients (4 examinations) were false-negative by 123I-MIBG SPECT/CT (median maximum diameter, 2.2 cm; range, 1.4–2.4 cm). Five of these lesions in 3 examinations (2 patients) led to a change of the therapeutic management (surgery). All positive lesions in both imaging procedures revealed true-positive lesions. The lesion-based sensitivity of 11C-HED PET was calculated to be 0.99 (80/81; 95% CI, 0.93–1.00); sensitivity of 123I-MIBG SPECT was calculated to be 0.93 (75/81; 95% CI, 0.84–0.97). Because there were no false-positive lesions, specificity could be stated to be 1.00 in both imaging techniques. Furthermore, examination-based sensitivity was 1.00 (19/19; 95% CI, 0.82–1.00) in both 11C-HED PET/CT and 123I-MIBG SPECT/CT.
Results of Imaging Procedures: Lesion-Based Analysis
SUVs in 11C-HED PET: Lesion-Based Analysis
The intensity of tracer uptake (4-value score) was variable in both 11C-HED PET/CT and 123I-MIBG SPECT/CT when comparing different patients or different lesions in the same patients (Figs. 2–4; Table 5). Whereas in most osseous lesions the intensity of 11C-HED uptake was equivalent (9/19; 0.47) or higher (8/19; 0.42) as compared with the 123I-MIBG uptake, in soft-tissue lesions tracer uptake was either equivalent (30/62; 0.48), higher using 11C-HED (18/62; 0.29), or higher using 123I-MIBG (14/62; 0.23).
A 33-y-old woman (patient HA) who had pheochromocytoma of right adrenal gland and resection years ago. 11C-HED PET/CT: (A and B) PET images; (C and D) PET/CT fusion images, 2 levels of coronal slices. There is local relapse (solid arrow) and metastases retrocrural (solid arrow), cervical (dotted arrow), and mediastinal (not shown) with highly increased tracer uptake. 11C-HED uptake of left adrenal gland is very low. 123I-MIBG SPECT/CT: (E and F) SPECT images; (G and H) SPECT/CT fusion images, 2 levels of coronal slices. There is moderately increased tracer uptake in local relapse (solid arrow) and physiologic uptake in left adrenal gland (open arrow). Retrocrural metastases are not visible with increased 123I-MIBG accumulation. Cervical and mediastinal metastases are not within field of view of SPECT.
A 57-y-old man (patient GP) with metastatic paraganglioma. Primary tumor (open arrows) in left paravertebral region with involvement of 10th thoracic vertebral body and osseous metastasis in right iliac bone (solid arrows) demonstrate moderately increased 11C-HED uptake in PET/CT: (A) PET, coronal slice; (B) PET/CT fusion image, coronal slice. 123I-MIBG SPECT/CT: (C) SPECT, coronal slice; (D) SPECT/CT fusion image, coronal slice. Very high tracer uptake is evident in primary tumor and only faintly increased tracer uptake is seen in osseous metastasis.
A 5-y-old girl (patient EN) who had neuroblastoma stage IV 3 y ago. Large local relapse in left upper abdomen does not demonstrate increased 11C-HED uptake above surrounding tissue in PET/CT: (A) PET, coronal slice; (B) PET/CT fusion, coronal slice; (C) PET, transversal slice; (D) CT, transversal slice; (E) gadolinium-enhanced T1-weighted MRI with fat saturation, transversal slice. Osseous metastasis (solid arrows in A and B) in left hemipelvis is visible with faintly increased tracer uptake. 123I-MIBG SPECT/CT: (F) SPECT, coronal slice; (G) SPECT/CT fusion, coronal slice. Local relapse (open arrows) and osseous metastasis (solid arrows) show highly increased 123I-MIBG accumulation. Neuroblastoma was confirmed histologically at first diagnosis 3 y ago. In relapse situation, osseous involvement was confirmed by bone marrow puncture. Additionally, patient showed increased urinary catecholamines at first diagnosis and in relapse situation.
Comparison of 11C-HED Uptake and 123I-MIBG Uptake: Lesion-Based Analysis
Lesions Outside Field of View of 123I-MIBG SPECT/CT.
In addition to the above lesions, the following lesions were found in regions outside the field of view of the 123I-MIBG SPECT/CT in a total of 10 pairs of examinations in 7 patients: 9 concordant positive lesions; 16 lesions true-positive using 11C-HED PET/CT and false-negative using planar 123I-MIBG scintigraphy; and 5 lesions true-positive using planar 123I-MIBG scintigraphy and not within the field of view of the 11C-HED PET/CT (1 scull, 2 distal femur, 2 tibia).
DISCUSSION
Several imaging procedures have been used to localize tumors of the sympathetic nervous system. CT and MRI provide excellent morphologic images and offer high sensitivities in the depiction of pheochromocytomas and neuroblastomas (1,11) but, unless indicated, only the abdomen is scanned and, therefore, small extraabdominal tumors may be missed. Moreover, CT and MRI depict only morphologic abnormalities and cannot functionally characterize adrenal or extraadrenal masses. Widely applied whole-body 123I-MIBG scintigraphy localizes neuroblastoma and pheochromocytoma with a high sensitivity and very high specificity and is extremely helpful in the detection of extraadrenal tumor sites (1). However, 123I-MIBG scintigraphy comes with some disadvantages, such as limited spatial resolution; limited sensitivity in small lesions; the need for 2 or—in the case of SPECT—even more acquisition sessions with the consequent delay between the start of the examination and result; and the relatively high radiation exposure.
Feasibility of Whole-Body 11C-HED PET/CT
In this study, 11C-HED PET/CT using the whole-body technique was introduced as an imaging tool for tumors of the sympathetic nervous system. The study data show that whole-body 11C-HED PET/CT is feasible in a clinical setting in all age group, including very young children. Similar to 18F-FDG PET/CT, in toddlers sedation as well as—in rare cases—general anesthesia has to be performed for scanning (12). Using a state-of-the-art PET/CT scanner excellent quality attenuation-corrected images were obtained.
Comparison of 11C-HED and 123I-MIBG
11C-HED PET/CT depicted more true-positive lesions compared with 123I-MIBG SPECT/CT and clearly more compared with planar 123I-MIBG scintigraphy. This led to a change in the therapeutic management (surgery and external radiotherapy) in 5 of 6 additional lesions in 3 of 4 examinations. Because of the limited number of patients there is a large overlap of the 95% CI of the calculated sensitivities of both tomographic methods. Therefore, 11C-HED PET has at least the same high sensitivity as 123I-MIBG SPECT. However, a large neuroblastoma relapse with high 123I-MIBG uptake did not accumulate more 11C-HED than the surrounding tissue, whereas osseous metastases in the same patient were positive in both imaging modalities. Furthermore, in 23% of soft-tissue lesions visually assessed 123I-MIBG uptake was more intensive than 11C-HED accumulation, whereas in 29% of soft-tissue lesions the uptake intensities were the other way round. Because the uptake mechanisms of both radiotracers are similar, an explanation could be distinct transport kinetics of both tracers in various tumors and in different tumor sites in the same patient depending on the host organ. This aspect is well known from the comparison of 123I-MIBG scintigraphy with image acquisition after 4 and 24 h following injection and 131I-MIBG scintigraphy with image acquisition several days after injection. Another explanation especially in small lesions may be the partial-volume effect that is more pronounced in SPECT than in PET. Further studies are necessary to elucidate this aspect. In accordance with the literature, specificity is very high, both using 11C-HED PET/CT and 123I-MIBG SPECT/CT, because of the specific uptake mechanism of both radiotracers (7,13).
Advantages of 11C-HED PET/CT
Compared with 123I-MIBG scintigraphy including SPECT/CT, 11C-HED PET/CT has several advantages: Because of the favorable physical conditions of positron emitters, PET enables higher spatial resolution than conventional radionuclide imaging methods. This higher spatial resolution in combination with the observed selective and distinct tracer accumulation in tumors of the sympathetic nervous system enables the acquisition of excellent-quality images of these tumor entities. The value of whole-body tomography obtained by PET in comparison with the limited field of view obtained by SPECT is obvious in patients with multiple metastases. The high image quality results in the detection of more and smaller lesions. Whereas 123I-MIBG scintigraphy and SPECT/CT require at least 18–24 h to achieve tumor-to-background ratios adequate for imaging, whole-body 11C-HED PET/CT is completed in 1 examination within 30 min after injection. 123I-MIBG scintigraphy requires at least 2 and in the case of SPECT up to 4 acquisition sessions are required. This advantage of 11C-HED PET/CT is especially beneficial for children where the shorter scanning time improves comfort as well as compliance and reduces sedation (or anesthesia) time. Whole-body and especially thyroid radiation exposure are considerably lower using 11C-HED PET (effective dose equivalent in adults, 1.2 mSv) compared with 123I-MIBG scintigraphy (effective dose equivalent in adults, 6.0 mSv) (5,14).
Disadvantages and Limitations of 11C-HED-PET/CT
However, 11C-HED PET/CT has also some disadvantages and limitations compared with 123I-MIBG scintigraphy. Because of the short half-life of 11C, an on-site cyclotron is necessary. Therefore, the availability is limited. Furthermore, the on-site production for every patient is time consuming and cost intensive. Although the present study has proven that 11C-HED PET/CT can be implemented in the clinical routine, the short half-life requires a rigid time schedule, which may be difficult in children or in patients who are in a poor clinical condition. Because of renal excretion during imaging, tumor manifestations next to the kidney or ureter may be missed using 11C-HED PET. In this study, this potential disadvantage was obviated by 11C-HED PET/CT fusion images. Furthermore, the relatively high physiologic liver uptake of 11C-HED may impede the detection of small liver metastases. Some of these shortcomings may be avoided by using PET/CT with 18F-labeled tracers (half-life, 110 min), such as 6-18F-fluoro-3,4-dihydroxy-2-phenylalanine (18F-DOPA), which has been used for imaging of a variety of neuroendocrine tumors (15–17). To our knowledge, there has been no study relating to 18F-DOPA PET in neuroblastoma and there are no comparison studies between 11C-HED PET and 18F-DOPA PET.
CONCLUSION
In summary, this study demonstrates that whole-body 11C-HED PET/CT is an imaging technique, with functional images of excellent quality, that provides high sensitivity and very high specificity in the detection of tumors deriving from the sympathetic nervous system. Whole-body scanning can be performed in a clinical setting in all age groups. 11C-HED PET/CT might be considered as the radionuclide imaging technique of choice if a cyclotron facility is available.
Acknowledgments
The authors gratefully acknowledge the assistance of Anika Brunegraf and all other technologists of the Department of Nuclear Medicine and the Department of Clinical Radiology in performing the scans as well as Monica Trub for preparing the 123I-MIBG. The authors also thank all radiologists of the Department of Clinical Radiology who have been involved in the interpretation of the low-dose CT scans and the additional morphologic imaging.
Footnotes
-
COPYRIGHT © 2006 by the Society of Nuclear Medicine, Inc.
References
- Received for publication March 16, 2006.
- Accepted for publication July 17, 2006.