Abstract
Cardiac 123I-labeled metaiodobenzylguanidine (MIBG) activity has significant incremental prognostic value, but the difference between the long-term prognostic value of MIBG imaging for ischemic cardiomyopathies and the long-term prognostic value of MIBG imaging for idiopathic cardiomyopathies is not clear. This study aimed to determine whether assessment of cardiac 123I-MIBG activities in ischemic and idiopathic cardiomyopathies have equally prognostic values and whether the kinetics are different because of the underlying etiologies. Methods: After quantitative 123I-MIBG imaging, 76 ischemic and 56 idiopathic cardiomyopathy patients were prospectively followed up for 54 mo. In addition to conventional parameters, cardiac 123I-MIBG activity was quantified as a heart-to-mediastinum ratio (H/M) for early and late images and the washout kinetics were calculated using tomographic imaging. The data were compared with those obtained from 16 healthy volunteers. Results: During follow-up, 29 deaths from heart failure, 11 sudden cardiac deaths, 2 deaths from arrhythmia, and 5 deaths from acute myocardial infarction were documented. Multivariate discriminant analysis using the Cox proportional hazards model showed that, in comparison with other variables, late H/M was the most powerful independent predictor of a lethal clinical outcome in ischemic (Wald χ2 = 18.6502; P = 0.0000) and idiopathic (Wald χ2 = 5.3394; P = 0.0208) groups. When patients with left ventricular ejection fraction (LVEF) < 40% were considered, late H/M had the greatest statistical power in both groups. Kaplan–Meier analysis showed late H/M to have an identical threshold (1.82) for both groups for identifying patients at risk of cardiac death. Likewise, when analysis was restricted to patients with an LVEF < 40%, the upper cutoff value of late H/M was 1.50 (P = 0.0358; log rank = 4.41) for ischemic patients and 2.02 (P = 0.0050; log rank = 7.86) for idiopathic patients. For patients with an LVEF < 40% and a late H/M less than the identified threshold of late H/M, the annual rate of cardiac death was greatest, 18.2%/y for the ischemic group and 11.9%/y for the idiopathic group. Conclusion: Cardiac 123I-MIBG activity has the most powerful independent long-term prognostic value for both ischemic cardiomyopathy patients and idiopathic cardiomyopathy patients, indicating that both disease processes have common pathophysiologic and prognostic implications of impaired cardiac sympathetic innervation. Although combined testing of cardiac function and 123I-MIBG activity is most likely to identify patients at increased risk of cardiac death, the underlying etiology of cardiac dysfunction may affect the threshold of 123I-MIBG activity for the differentiation of high-risk patients.
Both idiopathic cardiomyopathy (ICM) and ischemic cardiomyopathy (ISM) are widespread and major underlying cardiac diseases responsible for heart failure, the need for heart transplantation, or ultimately serious consequences. Current methods of assessing which patients from both groups are at highest risk and can benefit from aggressive therapeutic interventions are inadequate. Autonomic dysfunction has been shown to increase the risk of death in patients with heart disease and may be applicable to all patients with cardiac disease regardless of etiology (1–3). One way to assess myocardial autonomic function is with 123I-labeled metaiodobenzylguanidine (MIBG) imaging (3,4). Cardiac 123I-MIBG imaging has enabled direct evaluation of overall cardiac sympathetic function, including uptake, reuptake, storage, and release processes of norepinephrine at presynaptic nerve terminals, rather than real-time, beat-by-beat sympathetic drive (4). Cardiac 123I-MIBG markers that have been used include global myocardial uptake (heart-to-mediastinum ratio) (3), washout kinetics (5), and regional uptake heterogeneity (6). Our recent study revealed that cardiac 123I-MIBG activity, as measured by the heart-to-mediastinum ratio, has independent and incremental prognostic value (7). In contrast, increased 123I-MIBG washout has been observed not only in heart failure (5–10) but also in other cardiac conditions, such as overloaded, hypertrophied, coronary, and diabetic hearts (11–15), independent of underlying cardiac disease (16), and cardiac 123I-MIBG kinetics are a possible marker of responsiveness to drug interventions (17,18).
Imaging with 123I-MIBG has been shown to identify high risk in both patients with ICM and patients with ISM (3,7). Most of the previous studies on prognosis or drug intervention, however, predominantly used ICM patients as subjects. Whether cardiac 123I-MIBG activity is of long-term prognostic value in ISM as well as in ICM has not been determined. We believe that cardiac autonomic dysfunction may be a common endpoint leading to cardiac death, regardless of the underlying etiology of the cardiac disease. This study tested the hypothesis that cardiac 123I-MIBG imaging can help identify a high probability of cardiac death in patients with ICM and ISM.
MATERIALS AND METHODS
Patient Population
Of 205 consecutive patients who had a left ventricular ejection fraction (LVEF) < 50% and who underwent cardiac 123I-MIBG imaging between January 1993 and March 1995, 76 patients with ISM and 56 patients with ICM, having met the following criteria, were enrolled in the current study: a diagnosis of ISM or ICM based on the results of standard clinical examinations, including chest radiography, electrocardiography, stress testing, and 2-dimensional echocardiography; evaluation of left ventricular dimensions and function by 2-dimensional echocardiography and radionuclide ventriculography, respectively; a stable general condition throughout the examination protocol; a radionuclide LVEF of <50%; cardiac 123I-MIBG imaging within 1 wk (before or after) of cardiac function tests; follow-up of >1 y (up to 7 y) or until cardiac or noncardiac death; no indication for an invasive therapeutic procedure at the time of entry; and consent to participate in the study, as required by the ethics committee of Sapporo Medical University Hospital, Hokkaido Cardiovascular Hospital, and Sapparo Junkanki Clinic. In addition to left ventricular dysfunction, at least 3 of the following 4 findings were used for identifying the etiology of cardiomyopathy: clinical history of coronary artery disease and coronary risk factors; findings of ischemic ST-segment depression on resting and exercise-stress electrocardiography; findings of a fixed or reversible defect on resting or stress myocardial perfusion imaging with 201Tl, 99mTc-sestamibi, or 99mTc-tetrofosmin, performed on a separate day from 123I-MIBG imaging; and angiographic findings of multiple or diffuse stenotic lesions with luminal narrowing ≥ 75%. Idiopathic dilated cardiomyopathy was diagnosed if the patient had a clinical history of heart failure symptoms; a resting LVEF of <50%; no medical history or clinical findings of organic cardiac diseases that could explain symptoms and cardiac dysfunction, including coronary artery disease, congenital heart disease, valvular heart disease, metabolic disorders, endocrinologic disorders, and neuromuscular disorders; and no luminal coronary narrowing ≥ 50%. At the time that patients entered this study, none had undergone or was scheduled to undergo surgical or interventional procedures, including coronary artery bypass grafting, coronary angioplasty or stenting, implantation of cardioverter defibrillators, and heart transplantation; that is, all patients had selected conservative medical therapy. None of the patients had a history of acute ischemic events or acute myocardial infarction within the previous 6 mo. Sixteen healthy volunteers (9 males, 7 females; age range, 15–71 y; mean age, 55 ± 16 y) were used as control subjects to determine reference ranges of 123I-MIBG activity and washout kinetics (Table 1). None of the control subjects had organic cardiac disease; cardiac dysfunction; hypertension; or renal, endocrinologic, neurologic, or malignant disorders, and all had an LVEF ≥ 50%, fractional shortening ≥ 25%, and a left ventricular end-diastolic dimension < 55 mm.
Clinical and Scintigraphic Data for Study Population
There were 76 ISM patients (62 males, 14 females; mean age, 68 ± 12 y) and 56 ICM patients (43 males, 13 females; mean age, 60 ± 13 y). The mean age of the ISM patients was significantly (P < 0.001) higher than that of the ICM patients. The mean New York Heart Association (NYHA) functional class (2.4 ± 1.0) in the ICM group was significantly (P < 0.001) greater than that (1.8 ± 0.9) in the ISM group, and symptomatic heart failure at entry was documented in 40 (53%) of the ISM patients and in 43 (77%) of the ICM patients (P < 0.008). LVEF and left ventricular dimension at end-diastole (LVDd) in the ISM and ICM groups were similar: LVEF, 32% ± 11% and 30% ± 11%, respectively; LVDd, 57 ± 10 mm and 61 ± 13 mm, respectively (Table 1). An LVEF < 40% was found in 52 ISM patients (75%) and in 42 ICM patients (81%) when 11 patients with noncardiac death were excluded (Table 2). Use of nitrates was more frequent in the ISM patients, whereas the ICM patients used digitalis and angiotensin-converting enzyme inhibitors more frequently.
Clinical and Scintigraphic Data for Surviving and Cardiac Death Patients
Study and Follow-Up Protocols and Outcome Measures
Cardiac planar and SPECT imaging with 123I-MIBG was performed within 1 wk of cardiac function tests using radionuclide ventriculography with 740 MBq 99mTc-albumin and 2-dimensional echocardiography. Patients were subsequently followed prospectively by their primary physicians for at least 1 y or until the primary endpoint of the study (mortality) was documented. No patients underwent coronary intervention, ICD implantation, open-heart surgery, or heart transplantation during follow-up. Measures of outcomes were defined as mortality from any cause, including cardiac death from uncontrollable congestive heart failure or cardiogenic shock, uncontrollable arrhythmia, myocardial infarction, or sudden cardiac death (3,7). Detailed information on patient conditions and cause of death was directly collected from physicians at the medical centers at which patients were followed up or to which they were transferred. Sudden cardiac death was defined as an unexpected circulatory collapse without definite premonitory symptoms or signs and resulting in death within several hours after onset.
Scintigraphic Imaging and Quantification of Cardiac MIBG Activity
Planar and SPECT imaging was performed 30 min (early) and 3–4 h (late) after an intravenous injection of 111 MBq 123I-MIBG (Daiichi Radioisotope Labs, Ltd., Tokyo, Japan), with a specific activity of 1,665–2,035 MBq/mg, under resting and fasting conditions. Planar data were acquired from an anterior view using a gamma camera equipped with a low-energy, general-purpose collimator, with a 20% window centered on 159 keV. SPECT data were then acquired from a 45° left posterior oblique angle to a 45° right anterior oblique angle over a 180° arc in 36 preset sampling angles for 30 s per angle. The data were stored in a 64 × 64 matrix (7). After reconstruction of short-axis slices using a backprojection method with a Shepp–Logan filter, a polar map was generated to calculate washout kinetics of myocardial 123I-MIBG activity by avoiding contamination by background activity. In this study, however, no SPECT data could be obtained for 15 (20%) of the 76 ISM patients and for 6 (11%) of the 56 ICM patients because of substantially reduced (not visually identifiable) cardiac uptake of 123I-MIBG, even in early planar images. Therefore, for these patients, only data obtained from planar 123I-MIBG imaging were used for further analysis. For patients who had been prescribed oral drugs before imaging, drug administration was withdrawn transiently for at least a half-day and then was restarted after the study. As previously described (7), myocardial 123I-MIBG activity was quantified as a heart-to-mediastinum ratio (H/M) by manual setting of an 11 × 11 pixel region of interest on cardiac and upper mediastinal areas of early and late planar images by nuclear medicine staff without knowledge of the patient’s clinical data but with a highly reproducible technique of data sampling (y = 0.29 + 0.99x; r = 0.996; n = 40; P < 0.0001). When manual tracing was difficult because of absence of myocardial 123I-MIBG uptake, a planar perfusion image from an anterior view was used as a reference to identify the cardiac region (Fig. 1). Resting perfusion imaging with 201Tl (111 MBq), 99mTc-sestamibi (600 MBq), or 99mTc-tetrofosmin (600–740 MBq) was performed 10 min or 30–60 min after the tracer injection. Planar data were obtained from an anterior view using a gamma camera equipped with a low-energy, high-resolution, parallel-hole collimator (ZLC 75/Scintipac 2400 or ZLC 7500/Scintipac 700; Shimadzu, Tokyo, Japan, or Starcam 4000XC/T; General Electric Medical Systems, Milwaukee, WI), followed by SPECT imaging with the same protocol as that used for 123I-MIBG imaging. The washout rate of cardiac 123I-MIBG activity was calculated, using SPECT data and a polar map technique, as the percentage of change from early to late images without a physical decay correction: washout rate = [100 × (early 123I-MIBG activity − late 123I-MIBG activity)/early 123I-MIBG activity] (7). Briefly, depending on patient heart size, 12–16 short-axis slices were selected to generate early and late polar maps. For each pixel in the polar maps, washout rate was calculated using early and late 123I-MIBG data to obtain the mean value that was used as a washout rate for each patient.
Planar early and late 123I-MIBG and 201Tl images of 2 typical patients, 72-y-old man with ISM and sudden cardiac death (A) and 65-y-old woman with ICM and death from heart failure (B). Both early and late cardiac 123I-MIBG uptakes are tremendously reduced in well-perfused hearts; in particular, late H/M was <1.5 in both patients.
Assessment of Prognostic Variables and Statistical Analysis
The following clinical, echocardiographic, and scintigraphic variables were analyzed: age, sex, underlying cardiac disease (ISM or ICM), hypertension, diabetes mellitus, atrial fibrillation, ventricular tachycardia, NYHA functional class, LVDd and left ventricular dimension at end-systole (LVDs), fractional shortening, LVEF, H/M of 123I-MIBG activity 30 min (early H/M) or 3–4 h (late H/M) after injection, washout rate of 123I-MIBG activity, and medications used during follow-up (Table 1). Statistical values are shown as mean ± 1 SD. Prognostic values were determined using a statistical software package (SPSS Inc., Chicago, IL). Univariate and multivariate Cox proportional hazards regression analyses were used to identify predictors of survival; overall prognostic values were first analyzed using all deaths documented during the follow-up period, and then prognosis was analyzed more specifically using only cardiac deaths, of any cause, after exclusion of noncardiac deaths. Survival curves for subgroups of patients were created by the Kaplan–Meier method to clarify the time-dependent cumulative survival rate, and the curves were compared using the 2-sample log-rank test. The mean values were compared between 2 groups and among 3 groups using an unpaired Student t test and a 1-way ANOVA with Bonferroni adjustment, respectively, and, when needed, prevalences were compared using a χ2 test. P < 0.05 was considered statistically significant.
RESULTS
The mean follow-up intervals were 54 ± 31 mo for the ISM group and 55 ± 29 mo for the ICM group (Table 1). During follow-up, 47 cardiac and 11 noncardiac deaths were recorded (Table 1). In the ISM group, the cardiac deaths included 13 deaths from heart failure, 9 sudden cardiac deaths, 1 death from arrhythmia (uncontrollable ventricular tachycardia and fibrillation), and 5 deaths from myocardial infarction. In the ICM group, 16 deaths from heart failure, 2 sudden cardiac deaths, and 1 death from arrhythmia were documented. Figure 1 shows planar 123I-MIBG and 201Tl images of 2 typical patients from the ISM and ICM groups who died from cardiac conditions. Despite well-perfused myocardium (as determined by rest thallium, sestamibi, or tetrofosmin imaging), both early and late cardiac 123I-MIBG uptakes were considerably reduced to <1.5 as a late H/M. Although the ISM group had a greater prevalence of sudden cardiac death and death from arrhythmia (13%) than did the ICM group (5.4%), and the ICM group had a greater prevalence of death from heart failure (29%) than did the ISM group (17%) (Table 1), the differences were not statistically significant. The ISM and ICM groups had comparable early and late H/Ms of 123I-MIBG activity, but both of these were significantly (P < 0.05) lower than those of the control group: 1.95 ± 0.41 and 1.79 ± 0.38, respectively, for the ISM group; 1.91 ± 0.43 and 1.71 ± 0.41, respectively, for the ICM group; and 2.30 ± 0.17 and 2.38 ± 0.29, respectively, for the control group (Table 1). Compared with the control group, both the ICM group and the ISM group had a significantly greater washout rate of 123I-MIBG activity, and the washout rate of the ICM group was significantly greater than that of the ISM group: 42% ± 13% for the ICM group, 34% ± 13% for the ISM group, and 20% ± 9% for the control group (P < 0.001) (Table 1).
In the ISM group, patients who died from cardiac conditions had a significantly greater LVDd and LVDs, and a smaller percentage of fractional shortening, than did surviving patients (Table 2). In the ICM group, patients who died from cardiac conditions and those who survived had a comparable NYHA class and cardiac function (Table 2). In both the ISM and the ICM groups, patients who died from cardiac conditions tended to have a smaller H/M of 123I-MIBG activity than did patients who survived: 1.67 ± 0.34 and 1.84 ± 0.37, respectively (P = 0.057), for the ISM group; 1.56 ± 0.35 and 1.79 ± 0.40, respectively (P = 0.042), for the ICM group (Table 2). ICM patients who died from cardiac conditions, however, had a significantly greater washout rate (46% ± 10%) than did ICM patients who survived (38% ± 13%) and ISM patients with lethal cardiac events (31% ± 13%). For ISM patients, there was no significant difference in washout between those who survived and those who had cardiac death. Five ISM patients who died of acute myocardial infarction tended to have greater late MIBG activity (1.79 ± 0.39) than did ISM patients who died of pump failure (1.64 ± 0.34) or who had sudden cardiac death (1.66 ± 0.35), although the statistical powers were not significant (P = 0.715). There was also no significant difference in clinical variables, cardiac function, or other MIBG data between ISM patients who died of acute myocardial infarction or pump failure and ISM patients who had sudden cardiac death.
Table 3 summarizes the overall results of prognosis analysis for 69 ISM patients after 7 patients who had died from noncardiac causes had been excluded to more specifically identify the prognostic powers of clinical, scintigraphic, and cardiac function parameters. Multivariate discriminant analysis using the Cox proportional hazards model showed that late H/M was the most powerful independent predictor of a lethal clinical outcome, with a Wald χ2 value of 18.6502, an odds ratio of 0.0050, and a 95% confidence interval of 0.0004–0.0555 (P = 0.0000), compared with other significant variables, such as early H/M, atrial fibrillation, NYHA class, and the male sex (Table 3). Likewise, when 52 ISM patients with an LVEF < 40% were analyzed, late H/M had the greatest Wald χ2 value (16.9424), with an odds ratio of 0.0061 and a 95% confidence interval of 0.00054–0.0694 (P = 0.0000), compared with other significant variables (Table 4). Table 5 summarizes the overall results of prognosis analysis for 52 ICM patients after exclusion of 4 patients with noncardiac causes of death. Multivariate discriminant analysis revealed that late H/M was the only significant independent predictor of cardiac death for the 52 ICM patients (Wald χ2 value, 5.3394; odds ratio, 0.1326; 95% confidence interval, 0.0239–0.7359 [P = 0.0208]) and when analysis was restricted to 42 ICM patients with an LVEF < 40% (Table 5).
Multivariate Analysis with Cox Proportional Hazards Model for Prediction of Overall Cardiac Deaths in 69 Patients with Ischemic Cardiomyopathy When LVEF Was <50%
Multivariate Analysis with Cox Proportional Hazards Model for Prediction of Overall Cardiac Deaths in 52 Patients with Ischemic Cardiomyopathy When LVEF Was <40%
Multivariate Analysis with Cox Proportional Hazards Model for Prediction of Overall Cardiac Deaths in Idiopathic Cardiomyopathy
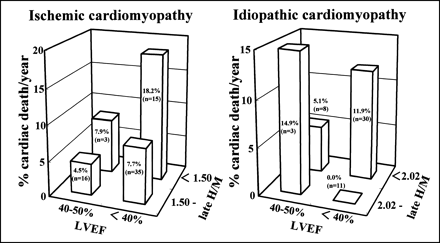
In the current population, several candidate thresholds of late H/M using the mean value and SD of late H/M in cardiac death patients (Table 2) were evaluated by Kaplan–Meier analysis to identify an upper limit of late H/M for differentiating patients who are at high risk from those who are not. For all patients, the upper cutoff values of late H/M were 1.82 (P = 0.0321; log rank = 4.59), which was the mean value plus 0.5 SD in ISM patients who died from cardiac conditions, and 1.82 (P = 0.0255; log rank = 4.99), which was the mean value plus 0.75 SD in ICM patients who died from cardiac conditions (Table 6). The 5-y survival rates of patients with versus those without a late H/M < 1.82 were 56% versus 83% for the ISM group and 51% versus 86% for the ICM group (Fig. 2). When only patients with an LVEF < 40% were considered, however, the upper limit of late H/M for identification of high-risk patients was 1.50 (P = 0.0358; log rank = 4.41) for ISM patients and 2.02 (P = 0.0050; log rank = 7.86) for ICM patients (Table 7). In this case, the 5-y survival rates of ISM patients with versus those without a late H/M < 1.50 were 40% versus 68% and the 5-y survival rates of ICM patients with versus those without a late H/M < 2.02 were 48% versus 100% (Fig. 2). Figure 3 shows the annual rate of cardiac death when each patient group was divided into 4 subgroups. Except for the ICM subgroup with an LVEF of 40% or more and a late H/M > 2.02, which had only 3 patients, the subgroups with the greatest annual rates of cardiac death—18.2%/y for the ISM group and 11.9%/y for the ICM group—were those with both an LVEF < 40% and a late H/M less than identified thresholds. However, in ICM patients with an LVEF < 40%, there was no significant difference in clinical or cardiac function variables between the subgroups with and without a reduced late H/M.
Kaplan–Meier survival curves produced by identified cutoff values of late H/M in patients with ISM (A) and ICM (B). In both ISM and ICM groups, patients with late H/M < 1.82, as identified by overall analysis, show significantly lower survival rates than those with late H/M ≥ 1.82. Furthermore, when patients with LVEF < 40% are considered, ischemic patients with late H/M < 1.50 and idiopathic patients with late H/M < 2.02 show significantly lower survival rates than each counterpart.
Annual rate of cardiac death when each group was divided into 4 subgroups based on LVEF of 40% and on identified thresholds of 123I-MIBG activity. For LVEF of <40% and late H/M less than identified thresholds (1.5 for ischemic and 2.02 for idiopathic), annual rates of cardiac death are greatest, 18.2%/y for ischemic group and 11.9%/y for idiopathic group, when subgroup with LVEF of 40% or more and late H/M > 2.02 are excluded because of small number of patients (n = 3).
Comparison of Upper Cut-Off Values for Separating Cardiac Death Patients into 2 Subgroups With and Without Poor Prognosis Using Late H/M When LVEF Was <50%
Comparison of Upper Cut-Off Values for Separating Cardiac Death Patients into 2 Subgroups With and Without Poor Prognosis Using Late H/M When LVEF Was <40%
DISCUSSION
Our study cohort comprised patients with ISM and ICM because these are the most prevalent cardiomyopathies responsible for cardiac death and heart transplantation. Of the clinical indices analyzed for both cardiomyopathies, cardiac 123I-MIBG activity had the strongest independent long-term prognostic value. Overall, the fact that the ability of late 123I-MIBG uptake to identify patients at the highest risk for cardiac death was comparable for the 2 cardiomyopathies strongly suggests that, for both, a lethal clinical outcome involves impaired cardiac sympathetic innervation.
We performed both 30-min and 3- to 4-h postinjection imaging in this study to assess the integrity of presynaptic nerve terminals and uptake 1 function (19–21) and to calculate 123I-MIBG kinetics. The 123I-MIBG washout rate may be not only a significant marker of cardiac sympathetic drive (5,10) or norepinephrine (22) but also useful for ascertaining the mechanism of reduced cardiac 123I-MIBG activity. Both ISM and ICM patients, particularly ICM patients with lethal outcomes, had significantly higher 123I-MIBG washout rates than did control subjects (Tables 1 and 2), supporting the previous findings of accelerated sympathetic tone or norepinephrine spillover in heart failure patients. Neither 123I-MIBG washout rate nor early 123I-MIBG activity was, however, shown to be a significant independent predictor in recent studies (7,23), probably because an early 123I-MIBG uptake reflects only integrity of presynaptic nerve terminals and uptake 1 function whereas late 123I-MIBG activity includes overall information about neuronal functions from uptake to release through the storage system at nerve terminals (4,19). Augmented cardiac sympathetic drive or 123I-MIBG washout rate is not specific for heart failure and does not necessarily result in a later 123I-MIBG defect (5–15). In this study, therefore, the late-phase 123I-MIBG abnormality was shown to be a most powerful and independent predictor for cardiac death.
Several mechanisms are likely to be responsible for a lethal clinical outcome from later reduction of cardiac 123I-MIBG activity. Cardiac sympathetic neurons can be anatomically lost because of ischemic injury or myocardial scarring and because of the degenerative cardiomyopathy process. Distal denervation induced by proximal neuron damage or myocyte damage has been observed in patients with myocardial ischemia or acute infarction (14,24–26). Uptake 1 function may be impaired at nerve endings, which are an ATP-requiring system, in failing hearts because intracellular ATP content is depleted in the failing or ischemic myocardium (20,26,27) and sympathetic nerves are more susceptible to ischemia than are myocytes (14,24,25). These possibilities can elucidate both initial and delayed cardiac 123I-MIBG defects without an increased 123I-MIBG release. Functional abnormality, such as altered 123I-MIBG kinetics, may also explain reduction of late cardiac 123I-MIBG uptake. The greater washout rate in ICM patients who died from a cardiac condition, in comparison with surviving ICM patients and ISM patients, is likely to have been responsible for the further reduction in late cardiac 123I-MIBG activity (Table 2). The possible mechanisms behind the augmented 123I-MIBG kinetics are impaired norepinephrine storage at the cardiac synapse, augmented spillover because of an accelerated sympathetic drive, and relatively impaired efficiency of the reuptake mechanism compared with neuronal release of norepinephrine (MIBG) (11,22). Thus, both initial deficits in cardiac sympathetic activity and concurrent functional abnormalities may be responsible for a considerable reduction in cardiac 123I-MIBG activity.
SPECT imaging was used in this study for precise calculation of washout kinetics without contamination by increased background activity. Lung 123I-MIBG activity decreases considerably over several hours independently of cardiac disease (28). Especially when initial cardiac 123I-MIBG uptake is markedly low, this value may be exaggerated. 123I-MIBG washout rate assessed by planar imaging may thus have been overestimated as a prognostic marker in other studies, even though 123I-MIBG washout kinetics could be a sensitive marker of cardiac sympathetic drive and therapeutic manipulations (17,18). When H/M of 123I-MIBG activity was <1.5, however, it was extremely difficult to see 123I-MIBG uptake well enough to reconstruct SPECT images. Considerably reduced 123I-MIBG uptake was observed in 21 patients (17%), preventing us from obtaining data on tracer washout and regional abnormalities. In this context, SPECT imaging may be less efficient than quantitative planar imaging for risk stratification in heart failure patients (3,7,23). 123I-MIBG kinetics may be more reliable as an index of augmented cardiac sympathetic nerve drive or impaired presynaptic vesicular storage at a relatively early or compensatory stage of heart failure, rather than as a long-term prognostic marker.
Despite careful selection of patients in this study, selection bias may not have been eliminated completely because ICM patients had greater functional abnormality and ischemic patients were older (Table 1). Clinical characteristics, such as age, sex, and coronary risk, and therapeutic strategies can also modify the timing and outcome of cardiac events in coronary artery disease. Nevertheless, multivariate analysis clearly showed that the prognostic value of impaired cardiac sympathetic function was identical in ISM and ICM patients in terms of the statistically independent and most powerful predictive value of late 123I-MIBG activity for identifying patients most at risk for cardiac death. Circadian patterns of autonomic nervous system function parallel the timing of ischemia, myocardial infarction, arrhythmia, and sudden cardiac death. Sudden death from either ischemic or nonischemic cardiac disease appears to have a similar timing in heart failure patients, irrespective of the underlying cardiac conditions (29–32). These findings and our current results strongly suggest that altered cardiac sympathetic function has pathophysiologic and prognostic implications in both ISM and ICM processes. The precise etiologic relationship between cardiac sympathetic innervation and cardiac death was not determined by this study, but several possible explanations can be offered. Impaired cardiac sympathetic innervation is closely related to hemodynamic and thrombogenic states leading to plaque rupture and thrombus formation, to arrhythmogenesis from sympathovagal imbalance and denervation supersensitivity (25), to endothelial dysfunction, and to downregulated adrenoceptor function responsible for impaired inotropic and chronotropic reserves (23,33–35). The upper limit of late H/M (1.82) for differentiating high-risk patients from others was identical for both the ISM and the ICM groups. However, when LVEF was <40%, the H/M threshold was higher for ICM patients (2.02) than for ISM patients (1.50) (Fig. 3). Although the precise mechanisms of these differences are not clear from this study, underlying cardiac function, as well as autonomic function abnormality, is likely to still modify ultimate clinical outcome, and impairment of cardiac sympathetic innervation may have more crucial implications for prognosis in ICM patients when cardiac function is severely depressed.
This study had several limitations. It provided long-term follow-up results using comparable patient numbers in ICM and ISM groups when compared with earlier studies (3,7,23). However, how the current results would apply to patients at high risk for cardiac death from other diseases and to patients treated with currently accepted therapies remains to be determined. This study was designed before angiotensin-converting enzyme inhibitors, β-blockers, and implantable cardioverter defibrillators came into general use in heart failure patients, as well as before social acceptance of heart transplantation in our country (36). The current data are limited but may reflect the true predictive value of the imaging technique for lethal cardiac events without the confounding effects of more aggressive treatment. Prognostic data from the technique should be used for developing more effective therapeutic interventions for high-risk patients (37,38). Our recent study revealed potential for combining the use of 123I-MIBG uptake and heart rate variability analysis to select patients at increased risk for sudden death (6). Cohen-Solal et al. (23) reported a close correlation between late 123I-MIBG uptake and peak-exercise oxygen uptake. Kaye et al. (39), using an invasive method, showed greater prognostic value for cardiac sympathetic innervation than for whole-body indices. Thus, altered cardiac sympathetic nerve activity appears not only to be a consequence of degenerative disease processes but also to have etiologic implications for lethal clinical outcomes. More work is needed to better understand the interactions between myocardial injury and noncardiac effects, such as neurohormonal markers (40), pulmonary function, and peripheral factors, responsible for fatal clinical outcomes. Finally, sudden cardiac death is possibly attributable to lethal arrhythmias (6,37,38). The precise mechanisms of sudden cardiac death and the correlation between cardiac 123I-MIBG activity and lethal arrhythmias, however, remain to be revealed.
CONCLUSION
Independent of cardiac function parameters and 123I-MIBG washout kinetics, impaired 123I-MIBG activity is the most powerful long-term predictor for cardiac death in both ISM and ICM patients. Combined assessment of cardiac function and the 123I-MIBG activity threshold is more likely to identify an increased risk of cardiac death in both ISM and ICM patients. These findings suggest that impaired cardiac presynaptic function has pathophysiologic implications in the occurrence of lethal cardiac events in both ISM and ICM patients. The underlying etiology of cardiac dysfunction, however, may affect the threshold of 123I-MIBG activity for the differentiation of high-risk patients.
Acknowledgments
The authors thank the staff of the Second Department of Internal Medicine (Cardiology), Sapporo Medical University School of Medicine, for their cooperation with clinical services. The authors also thank the staff of the Division of Nuclear Medicine and Radiology, Sapporo Medical University Hospital; Hokkaido Cardiovascular Hospital; and Sapporo Junkanki \E Clinic for their technical assistance.
Footnotes
Received Apr. 9, 2001; revision accepted Aug. 20, 2001.
For correspondence or reprints contact: Tomoaki Nakata, MD, PhD, Second Department of Internal Medicine (Cardiology), Sapporo Medical University School of Medicine, S-1, W-16, Chuo-ku, Sapporo 060-0061, Japan.