Abstract
Cardiac neuroimaging with 123I-metaiodobenzylguanidine (123I-MIBG) has been officially used in clinical practice in Japan since 1992. The nuclear cardiology guidelines of the Japanese Circulation Society, revised in 2010, recommended cardiac 123I-MIBG imaging for the management of heart failure (HF) patients, particularly for the assessment of HF severity and prognosis of HF patients. Consensus in North American and European countries regarding incorporation into clinical practice, however, has not been established yet. This article summarizes 22 y of clinical applications in Japan of 123I-MIBG imaging in the field of cardiology; these applications are reflected in cardiology guidelines, including recent methodologic advances. A standardized cardiac 123I-MIBG parameter, the heart-to-mediastinum ratio (HMR), is the basis for clinical decision making and enables common use of parameters beyond differences in institutions and studies. Several clinical studies unanimously demonstrated its potent independent roles in prognosis evaluation and risk stratification irrespective of HF etiologies. An HMR of less than 1.6−1.8 and an accelerated washout rate are recognized as high-risk indicators of pump failure death, sudden cardiac death, and fatal arrhythmias and have independent and incremental prognostic values together with known clinical variables, such as left ventricular ejection fraction and brain natriuretic peptide. Another possible use of this imaging technique is the selection of therapeutic strategy, such as pharmacologic treatment and nonpharmacologic treatment with an implantable cardioverter–defibrillator or cardiac resynchronization device; however, this possibility remains to be investigated. Recent multiple-cohort database analyses definitively demonstrated that patients who were at low risk for lethal events and who were defined by an HMR of greater than 2.0 on 123I-MIBG studies had a good long-term prognosis. Future investigations of cardiac 123I-MIBG imaging will contribute to better risk stratification of low-risk and high-risk populations, to the establishment of cost-effective use of this imaging technique for the management of HF patients, and to worldwide acceptance of this imaging technique in clinical cardiology practice.
Multicenter studies with 123I-metaiodobenzylguanidine (123I-MIBG) in North America, Europe, and Japan recently demonstrated the prognostic efficacies of this neuroimaging technique (1–3). Japan has 22 y of experience with 123I-MIBG imaging in clinical cardiology practice. Several single-center studies have clarified the clinical implications of cardiac 123I-MIBG imaging, which can depict noradrenaline uptake and release processes. In 1980, 131I-MIBG imaging was first applied to adrenal medullary imaging. In 1987, Daiichi Radioisotope Laboratory (Fujifilm RI Pharma Co., Ltd., at present) performed a clinical trial of 123I-MIBG (MyoMIBG) for imaging of the heart, after which the Japanese Ministry of Welfare (Ministry of Health, Labor and Welfare, at present) approved the clinical use of 123I-MIBG in cardiology practice (in 1992). During the next 2 decades, Japan had a robust clinical experience dealing with heart diseases, including ischemic heart disease, arrhythmia, idiopathic dilated cardiomyopathy, hypertrophic cardiomyopathy, and cardiomyopathies secondary to diabetes, renal failure, and other metabolic disorders. However, cardiac 131I-MIBG imaging has been performed most effectively for chronic heart failure (HF). Achievements in Japan are summarized in the Japanese Circulation Society’s Guidelines for Clinical Use of Cardiac Nuclear Medicine (JCS 2010; English digest version 2012) (4).
In the 1990s, 123I-MIBG imaging was applied to neurologic indications, such as Lewy body diseases, which include Parkinson disease, dementia with Lewy bodies, and pure autonomic failure. Since then, 123I-MIBG imaging has contributed to the effective identification of Lewy body involvement in the heart. Experiences in the field of neurology in the past decade and incorporation into Japanese guidelines for neurologic indications have facilitated the clinical use of 123I-MIBG in this field (5), resulting in official approval by Japanese social health insurance. In 1993, the use of 131I-MIBG in the field of oncology was approved. The clinical indications for 123I-MIBG include neuroblastoma and pheochromocytoma.
This review surveys a history of cardiac 123I-MIBG imaging, recent advances in standardization of this imaging technique, and major achievements in cardiology. In addition, the possible efficacies and future directions for clinical decision making in the management of HF are discussed.
NUMBER OF 123I-MIBG STUDIES IN JAPAN
The use of 123I-MIBG studies since 2000 is summarized in the report of a nationwide survey (“The Present State of Nuclear Medicine Practice”) by the Japan Radioisotope Association. The number of myocardial perfusion imaging studies with SPECT was about 300,000 per year, and approximately 40,000 studies were performed with 123I-MIBG. According to data from another survey, a 2002–2012 survey by the Japanese Society of Nuclear Medicine on adverse reactions to radiopharmaceuticals, the annual numbers of 123I-MIBG studies were 33,000 for cardiology and 4,000 for oncology, and the increase in 123I-MIBG studies from 2011 to 2012 was 12% (Fig. 1). The number of 123I-MIBG studies for HF was estimated to be approximately 10,000 per year. The use of myocardial perfusion SPECT has slightly decreased in recent years in Japan, but it is noteworthy that the use of 123I-MIBG imaging has been gradually increasing.
Number of 123I-MIBG studies performed since 2000 (A) and breakdown in 2012 (B) in Japan.
CLINICAL USE OF 123I-MIBG STUDIES LEADING TO JAPANESE NUCLEAR CARDIOLOGY GUIDELINES
In the field of cardiology, 123I-MIBG has been applied to ischemic heart disease, and high sensitivities for the detection of myocardial ischemia have been reported. After the early success of coronary revascularization in patients with acute coronary syndrome, salvaged myocardium could be visualized as denervated but viable tissue in an area at risk by 123I-MIBG SPECT (6,7). Cardiac 123I-MIBG imaging has also been used for the identification of repeated ischemia due to coronary artery spasm (8,9). Cardiac 123I-MIBG imaging may be useful for the detection of undetermined, unstable, or recurrent ischemia without a stress test. However, the low image quality and nonspecific abnormality of the inferior wall (low specificity) obtained with cardiac 123I-MIBG SPECT imaging limit the application of this imaging technique for coronary artery disease. Stress myocardial perfusion imaging and myocardial fatty acid metabolism imaging with 123I-β-methyliodophenyl pentadecanoic acid are more widely preferred for detecting myocardial ischemia or ischemia-related myocardial injury in Japan, on the basis of the Japanese Circulation Society’s guidelines for nuclear cardiology (4).
Cardiac 123I-MIBG imaging plays a unique and pivotal role in clinical HF practice. Although ischemic HF is the most common etiology of HF in western countries, in Japan nonischemic HF is more common (2). Nonischemic dilated cardiomyopathy has been one of the important applications of cardiac 123I-MIBG imaging since the 1990s in Japan, although it is also important in North America and Europe (10–12). Regardless of HF etiology, reduced cardiac 123I-MIBG activity, quantified as the heart-to-mediastinum ratio (HMR), has been shown consistently to indicate poor cardiac survival. As discussed later, cardiac 123I-MIBG imaging can evaluate the pharmacologic effects of inhibitors of β-adrenoceptor function and the renin-angiotensin-aldosterone system, showing good efficacies of these drugs parallel to improvements in the HMR and 123I-MIBG washout rate (WR) in responders. Hypertrophied myocardium has reduced 123I-MIBG activity relative to perfusion tracer uptake together with increased 123I-MIBG clearance in patients with hypertrophic cardiomyopathy (13). A diabetic heart is also likely to have impaired 123I-MIBG activity (low HMR and 123I-MIBG defect) in association with disease progression (14). Table 1 summarizes major investigations that prospectively monitored patients with chronic HF for more than 2 y with an endpoint of cardiac death (15–25).
123I-MIBG Prognostic Studies in Japan with Endpoint of Cardiac Death
Table 2 shows pooled or multicenter analyses in Japan, North America, and Europe. On the basis of the clinical applications of 123I-MIBG and literature from Europe, North America, and Japan, the Japanese Circulation Society published the Guidelines for Clinical Use of Cardiac Nuclear Medicine in 2005 and revised them in 2010 by reviewing recent achievements (Table 3) (4).
Pooled or Multicenter Analyses in Japan, North America, and Europe
Recommendations for 123I-MIBG Sympathetic Imaging in Japanese Circulation Society’s Guidelines (4)
RECOMMENDED PROTOCOLS IN JAPAN
Acquisition Protocol
An 123I-MIBG scan is performed 15–30 min (early) and 3–4 h (late) after the tracer injection. A commonly used dose of 123I-MIBG in Japan is 111 MBq; this dose is lower than the recommended dose (111–370 MBq) in the United States and Europe (26). A planar image is obtained from an anterior view for 3–10 min with an energy window centered at 159 keV and a window width of 20% or 15%. When possible, tomographic data are subsequently acquired for differential diagnosis and localization of 123I-MIBG defects in coronary artery disease and neurodegenerative disorders, such as Lewy body diseases (5). Cardiac 123I-MIBG activity is affected by imaging conditions, particularly the collimator type; the HMR obtained with a medium-energy (ME) collimator is greater than that obtained with a low-energy (LE) one.
Parameters of 123I-MIBG Studies
The HMR is the most widely used 123I-MIBG parameter for the measurement of whole myocardial activity. A cardiac region of interest (ROI) is set manually over the heart without overlapping lung and liver activities and with a rectangular mediastinal ROI as a background (26). The reproducibility of the HMR is good within an institution (27), although variability can be observed, depending on the selection of the ROI size and location and the operator’s experience. Recently, software (smartMIBG; Fujifilm RI Pharma Co., Ltd.) became available for semiautomatic ROI settings and calculations of the HMR and WR (28). The software algorithm uses a circular heart ROI and a mediastinal ROI with a 10% width of the body and a 30% height of the mediastinum. The HMR is calculated as the average heart count per pixel divided by the average mediastinal count per pixel. The WR is also calculated for evaluating sympathetic tone or drive as follows: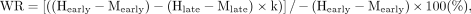 where Hearly and Hlate are average heart counts and Mearly and Mlate are average mediastinal counts in early and late scans, respectively. The coefficient k is a time decay correction factor of 1/0.5t/13 for time t (hours), and if the interval between the scans is 3 h, then k is 1.17. Ideally, the tracer kinetic (WR) can be estimated precisely by use of both background and physical decay corrections. However, these corrections are not necessarily performed routinely, and the WR in the previous literature should be carefully interpreted in this context. Although no background subtraction may be used for less variability (27), our recommendation is to use background subtraction for consistency among various studies other than HF.
where Hearly and Hlate are average heart counts and Mearly and Mlate are average mediastinal counts in early and late scans, respectively. The coefficient k is a time decay correction factor of 1/0.5t/13 for time t (hours), and if the interval between the scans is 3 h, then k is 1.17. Ideally, the tracer kinetic (WR) can be estimated precisely by use of both background and physical decay corrections. However, these corrections are not necessarily performed routinely, and the WR in the previous literature should be carefully interpreted in this context. Although no background subtraction may be used for less variability (27), our recommendation is to use background subtraction for consistency among various studies other than HF.
Regional Versus Global and Planar Versus SPECT Assessments
SPECT imaging can assess regional 123I-MIBG defects, which indicate viable but denervated, or injured, myocardial tissue. The Japanese Society of Nuclear Medicine (JSNM) Working Group database is the first 123I-MIBG SPECT database created for 180° and 360° rotations in each sex (Fig. 2) (29). However, there are several limitations of SPECT imaging. First, even in “nearly normal” subjects, inferior wall activity is often decreased, probably because of physiologic changes brought about by aging. Second, when cardiac 123I-MIBG activity is globally and markedly reduced, as it often is in advanced HF, reconstruction of SPECT images and regional assessment with a scoring system are difficult to achieve. Third, in a highly dilated heart, nonspecific inferior wall defects are observed, probably because of attenuation artifacts. Finally, inferior wall defects are also observed in diabetic hearts. Thus, although the regional assessment of 123I-MIBG distribution with high-quality imaging is useful for the detection of localized denervation, it seems to be supplementary to the global assessment of 123I-MIBG activity in HF.
Normal values for HMR (A) and WR (B) and polar maps (C) based on JSNM Working Group database. Italic numerals in bars indicate reference ranges. BG = background; LME = low to medium energy.
NORMAL VALUES AND STANDARDIZATION
Normal Values
Standardization of the HMR and WR is necessary for setting normal values and optimal thresholds for risk stratification. In a survey of 12 sources from the literature in Japan from 1994 to 2007, the means of early and late HMRs in the control (“normal”) groups ranged from 1.88 to 2.87 and from 1.84 to 2.49, respectively (Supplemental Data [supplemental materials are available at http://jnm.snmjournals.org]). In the “normal” JSNM Working Group database, early and late HMRs are 2.39 ± 0.21 (mean ± SD) and 2.49 ± 0.25 for the LE collimator and 2.76 ± 0.31 and 3.01 ± 0.35 for the ME collimator, respectively (29). Similarly, in 11 studies in Europe and the United States, the late HMR ranged from 1.77 to 2.50 (Supplemental Data). Figure 2 shows mean normal HMRs obtained with each collimator and the standardized HMR from the JSNM Working Group database (n = 62).
Standardization of HMR for Prognostic Evaluation
There are large variations in HMRs, depending on the scintillation camera, collimator, administration dose and specific activity of 123I-MIBG, and imaging protocol. In particular, high-energy photons in 123I, particularly the 529-keV photon (1.4%), result in numeric differences in measurements from LE- and ME-collimated images. Therefore, a dichotomous manner of risk assessment (low risk vs. high risk) with an HMR threshold may be questionable (30). However, it is crucial and possible to standardize 123I-MIBG parameters (HMR and WR) for clinical application in the management of HF. Figure 3 shows one of the promising processes for the appropriate use of 123I-MIBG parameters (i.e., HMR) in clinical decision making for patients with chronic HF.
Standardization of cardiac 123I-MIBG activity quantified as HMR by conversion of data obtained with low- to medium-energy (LME) collimator to data obtained with LE high-resolution (LEHR) collimator. (A) 32-y-old male with 22% LVEF and NYHA class 3. His HMR was converted from 1.36 obtained with LME collimator to 1.23 obtained with LEHR collimator, and 5-y mortality rate was reestimated to be more than 50% (10%/y). (B) 86-y-old woman with 51% LVEF and NYHA class 2. Her HMR was converted from 2.33 to 1.88, and 5-y mortality rate was recalculated to be 10% (2%/y).
We proposed a calibration phantom method to cross-calibrate HMRs among institutions (31,32). Because HMRs from 2 camera acquisition conditions have an approximately linear relationship, a conversion formula for 2 systems can be determined with the cross-calibration phantom designed for planar imaging. With this calibration method, the coefficient of conversion from an institutional HMR to the mathematically calculated reference value was measured in 225 experiments in 84 hospitals. The measured HMR was successfully converted to the standardized HMR among institutions. Our proposal was to use the standardized HMR comparable to that obtained with the ME collimator, which is most fitted for the 123I-tracer currently available for heart and brain studies. The standardization of the HMR significantly improved risk classification on the basis of the HMR either with LE- or ME-type collimators (32). The HMRs published in several studies in the literature also can be changed for specific conditions. Given that the ADMIRE-HF study showed that the HMR threshold was 1.6 when the LE high-resolution collimator was used (1), the threshold can be converted to 2.0 for an institution in which the ME general-purpose collimator is used. Figure 4 shows the results of data conversion and incorporation of the standardized HMR into the mortality risk model (2,33). This method enables the calibration of data obtained for any kind of HMR (either ME or LE collimator), contributing to the universal application and comparison of HMRs in the selection of a therapeutic strategy.
Process of standardization of cardiac 123I-MIBG data for calculation of HMR, risk stratification, and risk-based decision making in management of chronic HF. H = heart; M = mediastinum.
INCREMENTAL CLINICAL BENEFITS OF CARDIAC 123I-MIBG IMAGING FOR HF
Several studies in the literature have demonstrated independent and incremental prognostic values of cardiac 123I-MIBG imaging for patients with chronic HF in combination with clinical information, such as a history of myocardial infarction, New York Heart Association (NYHA) functional class, left ventricular ejection fraction (LVEF), plasma brain natriuretic peptide (BNP) level, and coexisting noncardiac conditions (such as diabetes mellitus, impaired kidney function, and anemia) (2,10,15,19,20,25,34–36). The prognostic value has been shown irrespective of the etiologies (ischemic or nonischemic) of HF and LVEF (Fig. 5) (35). Cardiac 123I-MIBG imaging can help cardiologists risk-stratify patients, select therapeutic strategy, and predict long-term survival of patients with chronic HF more precisely. Recent results from multicenter studies (1–3) strengthened the previous findings, demonstrating that cardiac 123I-MIBG imaging can be used—independently of conventional prognostic markers—to risk-stratify patients with HF (low risk vs. high risk for lethal events) and predict the probability of long-term survival with pharmacologic or nonpharmacologic treatments.
Cumulative mortality curves comparing patients with idiopathic dilated cardiomyopathy (A) and coronary artery disease (B) when cutoff values of 1.70 for HMR and 35% for LVEF were used with Japanese pooled databases (2). EF = ejection fraction.
PHARMACOLOGIC TREATMENT AND CARDIAC 123I-MIBG IMAGING
Because they result in definitive improvements in mortality, β-blockers and renin-angiotensin-aldosterone system inhibitors are widely accepted in patients with asymptomatic and symptomatic chronic HF. However, risk reduction rates for cardiac mortality with these medications are still limited to approximately 20%–30%. In real-world practice, some patients with chronic HF do not necessarily meet the entry criteria used in major drug intervention studies for HF or often have coexisting or unexpected noncardiac diseases that can affect clinical outcomes. Some patients with chronic HF cannot sufficiently benefit from these drug treatments or experience intolerance or adverse effects of the drugs, and physician preference sometimes results in underuse or underdosing of these drugs. Therefore, it is highly desirable to establish a method to appropriately identify patients who will exhibit a sufficient response and can tolerate contemporary drug therapy beyond physician preference or experience. Although only a few studies on this topic have been conducted (11,37–46), cardiac 123I-MIBG imaging can be used to monitor the effects of treatment with β-blocking agents, renin-angiotensin-aldosterone inhibitors, or their combinations by correlating an increase in cardiac 123I-MIBG activity (HMR) and a decrease in 123I-MIBG WR with an improvement in NYHA functional class, LVEF, or exercise tolerance.
Despite these data, it is more crucial to predict therapeutic efficacy and outcome improvement before the initiation of a drug intervention. Patients with a preserved cardiac HMR of 1.8 or more were shown to be tolerant of a metoprolol titration dose, and the findings were more likely related to the subsequent improvement in cardiac function during a 3-mo interval together with a reduction in the plasma noradrenaline concentration (39). In 167 patients with chronic HF (45), treatment with angiotensin-converting enzyme inhibitors or β-blockers significantly reduced cardiac death prevalence and the 5-y cardiac mortality rate compared with treatment without these drugs (15% vs. 37% and 21% vs. 42%, respectively; P < 0.05), and the risk reduction rate at 5 y in patients with an HMR of 1.53 or more was significantly greater than that in patients with an HMR of less than 1.53 and receiving the same drug treatment (67% vs. 32%, respectively; P < 0.05). Thus, current optimal drug treatment can improve survival rate, but the efficacy for clinical outcomes is likely to depend on cardiac 123I-MIBG activity, strongly suggesting that cardiac 123I-MIBG activity not only can estimate drug effects but also can predict cardiac risk improvement with appropriate drug treatment.
NEED FOR NEW RISK STRATIFICATION METHOD FOR DEVICE TREATMENT
There are inherent limitations in contemporary drug treatment; that is, some patients will be nonresponders from the outset, and some will cease to respond to contemporary drug therapy during a clinical course. Nonpharmacologic device treatment has evolved to definitively improve symptoms, the quality of life, and outcomes for such patients with refractory HF. An implantable cardioverter–defibrillator (ICD) can ablate lethal ventricular tachyarrhythmias and reduce sudden cardiac death (SCD) risk. Cardiac resynchronization therapy (CRT) with biventricular pacemakers can effectively reduce recurrent hospitalization and mortality risk in patients with prominent left bundle branch block and advanced systolic HF refractory to optimal drug treatment. CRT combined with ICD (CRTD) can reduce all-cause mortality, cardiac death, and recurrence of symptom aggravation and hospitalization in patients with advanced HF of NYHA class 3 or 4 and severe intraventricular dyssynchrony (47). In addition to the secondary prevention of SCD or lethal arrhythmic events, the most accepted ICD indication for the primary prevention of SCD is based on chronic HF presenting with prior myocardial infarction, NYHA class 2 or 3, and LVEF of 35% or less.
As introduced in major guidelines, the continuous increase in the numbers of patients and the robust amount of evidence relating to the efficacies of device therapy have facilitated prophylactic use of ICD, CRT, and CRTD in Japan as well as other developed countries. However, it is known that a large percentage of ICD devices are unlikely to deliver appropriate therapy during their lifetime, and nearly one-third of patients ineligible for an ICD (LVEF of >35%) die of SCD. Likewise, the clinical efficacies of CRT are limited in patients who have mild to moderate chronic HF (NYHA class 1 or 2), do not have a prolonged QRS duration of greater than 120 ms, or do not have reduced LVEF. Some patients cannot respond adequately to or may be ineligible (at a really low risk) for device treatment even when they meet currently available standard indication criteria. Conversely, even patients outside the indication criteria may die of SCD (consequently at a high risk) and are eligible for treatment. Besides device-related problems, the increasing need for medical resources, which are becoming limited, heightens the need to establish more appropriate identification—beyond that provided by conventional clinical markers—of patients who have chronic HF and are most likely or unlikely to benefit from device treatment in a cost-effective fashion (48–51).
DEVICE TREATMENT AND CARDIAC 123I-MIBG IMAGING
The Department of Japanese Government Social Insurance officially approved ICD use in 1996, CRT use in 2004, and CRTD use in 2006. Thereafter, several small but important studies (22,52–58) showed that excess activation of cardiac sympathetic nerve function and impaired cardiac sympathetic innervation, as assessed with cardiac 123I-MIBG imaging, are associated with arrhythmogenicity—leading to lethal ventricular arrhythmias, ICD shock against lethal arrhythmia events, and SCD independent of clinical, electrophysiologic, and LVEF symptoms (59). In addition to the assessment of BNP, LVEF, and myocardial viability, cardiac 123I-MIBG activity is used for the assessment of prognosis and the selection of therapeutic strategy at our institutes (56,57). The incremental prognostic value of this imaging technique is also supported by larger studies (60,61) and by a PET study with 11C-metahydroxyephedrine (62). Cardiac 123I-MIBG imaging has additive value for clinical information assessed with the Seattle Heart Failure Model in candidates at high risk for ICD, CRT, or CRTD (61), and death caused by arrhythmias or appropriate ICD discharge for lethal ventricular arrhythmias correlates with amounts of denervated myocardium (62). In response to CRT, cardiac 123I-MIBG activity improves together with symptomatic and functional improvements, and baseline cardiac 123I-MIBG activity correlates with CRT effects (63–66). More recently, cardiac 123I-MIBG activity was shown to be closely associated with mechanical dyssynchrony, as assessed with a speckle-tracking strain technique, and an HMR of 1.6 on 123I-MIBG studies is likely to be a cutoff value for predicting responses to CRT and long-term outcomes for dyssynchrony in Japanese patients (67). Thus, cardiac 123I-MIBG imaging enables cardiologists to help identify patients who are most susceptible to lethal arrhythmias and event risks and who can actually benefit most from device therapy by overcoming the limitations of current device therapy criteria, most of which consist of surrogate markers of lethal events, such as symptoms (NYHA class), clinical backgrounds, LVEF, and QRS prolongation (intraventricular electrical dyssynchrony).
CARDIAC 123I-MIBG IMAGING IN HEART TRANSPLANTATION
Heart transplantation for patients with terminal HF improves survival rates at 1 and 5 y, up to nearly 90% and 70%, respectively. However, because of a limited number of heart donors, the precise indication, the order of priority, and the appropriate timing of surgery are crucial clinical issues. Historically, because of a delayed national consensus on this treatment and because there are few donors, Japan has much less experience with heart transplantation per se than other countries and, therefore, has no significant clinical data on cardiac 123I-MIBG imaging for this treatment. Nevertheless, compared with other, standard parameters, cardiac 123I-MIBG imaging may contribute to improvements in determining the necessity of heart transplantation and expected survival interval (3,12,68,69) in an era when advanced device therapy, optimal drug treatment, and cardiac 123I-MIBG imaging are available. Cardiac 123I-MIBG imaging may be also useful for the assessment of reinnervation in transplanted hearts. Cardiac neuroimaging with 11C-metahydroxyephedrine or 123I-MIBG identifies ventricular sympathetic reinnervation (70–72), which slowly develops from the cardiac base several months after surgery and is observed in 40% of heart transplant patients 1 y after surgery (73). Although the clinical implications and mechanisms of the cardiac reinnervation process are not necessarily revealed, the restoration of cardiac sympathetic innervation is likely to increase exercise capacity by improving the blunted physiologic responses of heart rate and contractile function to exercise in patients with heart transplants (73). Assessment of the cardiac reinnervation process with cardiac 123I-MIBG imaging may be useful for the management of patients with heart transplants in an outpatient care unit by determining the appropriate exercise prescription, evaluating the exercise training effect and, hopefully, predicting an improvement in long-term survival.
IDENTIFICATION OF LOW-RISK PATIENTS WITH HF
Cost-effective treatment is generally a risk-based selection of therapeutic strategy. Precise identification of patients at low risk for lethal outcomes can contribute to the appropriate use of medical resources by minimizing diagnostic examinations, selecting a low-cost but effective treatment appropriately, and restraining from overuse of high-cost interventions in patients who are not likely to benefit from high-cost, invasive treatments. Previous investigations showed that the cutoff value for differentiating high-risk patients from low-risk patients likely is approximately 1.60–1.75 (1–3,15). Furthermore, the recent multicenter results for 600 to 1,300 patients with chronic HF (1–3) definitively demonstrated the ability of cardiac 123I-MIBG imaging to identify—independently of conventional prognostic markers—low-risk patients who could survive for several years (Fig. 6). In Japanese studies (2,33), annual all-cause mortality was less than 2% in patients with an HMR of greater than or equal to 2.0, and the mortality rates at 5 y were nearly 8% in patients with an HMR of greater than or equal to 2.0 and 10% to 15% in patients with an HMR between 1.7 and 2.0. Likewise, in the European study (3), mortality rates at 5 y were less than 3% in patients with an HMR of greater than or equal to 1.76 and nearly 15% in patients with an HMR between 1.33 and 1.75. Of note is that the HMR cutoff values among the studies were different (1–3) because of differences in patient backgrounds and, more importantly, because of technical differences in cardiac 123I-MIBG imaging (32). Nevertheless, cardiac 123I-MIBG activity quantified as HMR correlated consistently with survival rate for periods of 5 y or more over a wide range of HMRs—from less than 1.1 to 2.1 or more (2). Thus, these findings and recent advances in the standardization of cardiac 123I-MIBG imaging presented in this article support the clinical use of the quantitative 123I-MIBG parameter (HMR) for defining a low risk for lethal events over 5 y (33), for differentiating high-risk patients from low-risk patients, and for anticipating survival time in each patient with chronic HF.
Low risk of mortality in subjects with HMR of greater than 2.0. (A) All-cause mortality curves over 10 y for Japanese pooled databases (n = 1,322) (2), indicating low probability of lethal events independent of LVEF when HMR on 123I-MIBG studies was more than 2.0. (B) Cardiac mortality curves at 5 y, estimated with logistic model of HMR on 123I-MIBG studies (n = 933) (33), indicating low probability of lethal events independent of age difference when HMR on 123I-MIBG studies was more than 2.0.
LIMITATIONS AND FUTURE DIRECTIONS OF CARDIAC 123I-MIBG IMAGING
A growing body of evidence for cardiac 123I-MIBG imaging demonstrates great potential for helping in the selection of patients who are most eligible for advanced contemporary treatment rather than treatment through conventional methods. However, further investigations are needed to strengthen earlier findings and to provide reassurance for precise risk stratification and decision making in the selection of nonpharmacologic device treatment, including the prediction of responsiveness to the treatment. In the future, the increasing number of patients with chronic HF will outpace medical resources, and the application of device or heart transplantation treatment in patients who are at lower risk or who are less likely to benefit sufficiently from the treatment will be limited. More experience in cardiac 123I-MIBG imaging is needed to improve negative and positive predictive values for better differentiation of low-risk patients from high-risk patients, which will contribute to the effective use of medical resources. The utility of this imaging technique is not obvious enough for recommendation into international guidelines in other countries, unlike in Japan; however, even in Japan, data relating to cost-effectiveness and limited availability in cardiology practice are still insufficient. Future large-scale prospective multicenter studies would establish a practical and cost-effective use of cardiac 123I-MIBG imaging in combination with clinical information for patients with chronic HF to help clinicians optimize patient care (Table 4).
Current Tentative Clinical Use of Cardiac 123I-MIBG Imaging for HF
DISCLOSURE
This work was partly supported by the working group activity of the Japanese Society of Nuclear Medicine and by Grants-in-Aid for Scientific Research in Japan (to Kenichi Nakajima). Kenichi Nakajima has done collaborative research with Fujifilm RI Pharma Co., Ltd., which supplies 123I-MIBG in Japan. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Arnold Jacobson, MD, for comments on the article and Ronald G. Belisle for editorial assistance.
Footnotes
↵* Contributed equally to this work.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication September 30, 2014.
- Accepted for publication November 10, 2014.













