Abstract
123I-metaiodobenzylguanidine (123I-MIBG) has independent prognostic value for risk stratification among heart failure patients, but the use of concomitant medication should not affect its quantitative information. We evaluated whether the 4 classes of antidepressants currently most prescribed as first-line treatment for major depressive disorder (MDD) have the potential to alter 123I-MIBG imaging results. Methods: The inhibition effect of desipramine, escitalopram, venlafaxine, and bupropion on 131I-MIBG uptake was assessed by in vitro uptake assays using human neuroblastoma SK-N-SH cells. The half-maximal inhibitory concentration of tracer uptake was determined from dose–response curves. To evaluate the effect of intravenous pretreatment with desipramine (1.5 mg/kg) and escitalopram (2.5 or 15 mg/kg) on 123I-MIBG cardiac uptake, in vivo planar 123I-MIBG scanning of healthy New Zealand White rabbits was performed. Results: The half-maximal inhibitory concentrations of desipramine, escitalopram, venlafaxine, and bupropion on 131I-MIBG cellular uptake were 11.9 nM, 7.5 μM, 4.92 μM, and 12.9 μM, respectively. At the maximum serum concentration (as derived by previous clinical trials), the inhibition rates of 131I-MIBG uptake were 90.6% for desipramine, 25.5% for venlafaxine, 11.7% for bupropion, and 0.72% for escitalopram. A low inhibition rate for escitalopram in the cell uptake study triggered investigation of an in vivo rabbit model: with a dosage considerably higher than used in clinical practice, the noninhibitory effect of escitalopram was confirmed. Furthermore, pretreatment with desipramine markedly reduced cardiac 123I-MIBG uptake. Conclusion: In the present in vitro binding assay and in vivo rabbit study, the selective serotonin reuptake inhibitor escitalopram had no major impact on neuronal cardiac 123I-MIBG uptake within therapeutic dose ranges, whereas other types of first-line antidepressants for MDD treatment led to a significant decrease. These preliminary results warrant further confirmatory clinical trials regarding the reliability of cardiac 123I-MIBG imaging, in particular, if the patient’s neuropsychiatric status would not tolerate withdrawal of a potentially norepinephrine-interfering antidepressant.
- depression
- 123I-MIBG
- antidepressant
- cardiac sympathetic nerve system
- major depressive disorder
- myocardial sympathetic innervation imaging
Providing a semiquantitative score for mortality risk stratification in heart failure, the guanethidine analog 123I-metaiodobenzylguanidine (123I-MIBG) has recently been Food and Drug Administration–approved after extensive early-phase use (1–3). Sharing similar pathways with norepinephrine, including entering adrenergic cells through the uptake-1 pathway and being stored in presynaptic vesicles, the pharmacokinetics of 123I-MIBG depend mainly on norepinephrine-recycling tissues (4,5). However, the use of prescribed or over-the-counter medications has potential risks in patients being considered for cardiac 123I-MIBG studies. For example, the medication may artificially lower uptake, leading to misinterpretation of cardiac innervation status, or undesirable clinical consequences may ensue if the medication is withdrawn (6). Hence, the referring physician must carefully weigh the potential harm of withdrawing concomitant medications versus the potential benefit of accurate sympathetic nerve assessment by 123I-MIBG imaging (6,7).
Widespread availability of safer antidepressant classes has led to a significant increase in antidepressant prescriptions over the last few years. Instead of referring patients for cognitive-behavioral therapy, primary care providers in the United States prescribe double the total number of antidepressants that psychiatrists do (8,9). Because of a safety profile superior to that of the previously used tricyclic antidepressants (TCAs), 3 classes of next-generation antidepressants were recommended by a work group on major depressive disorders (MDDs) as optimal for first-line treatment in patients with MDD: selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and norepinephrine-dopamine reuptake inhibitors (NDRIs) (10,11).
The more extended use of 123I-MIBG imaging outside controlled clinical trials (1,12,13) and the potential of these drugs to interfere with norepinephrine transporters (NETs) (14–17) raise concern about inhibitory effects on cardiac 123I-MIBG uptake (6). It was previously established that TCAs and SNRIs significantly reduce 123I-MIBG uptake (4,18). However, substantial evidence is still lacking on whether other first-line antidepressants, such as SSRIs and NDRIs, interfere with NETs (6). Using an in vitro binding assay and an in vivo rabbit model that mimics uptake 1–mediated norepinephrine clearance comparable to the human heart (19), we explored the potential of the 4 antidepressant classes most prescribed for MDD to alter 123I-MIBG imaging results.
MATERIALS AND METHODS
Radiopharmaceuticals
123I-MIBG (specific activity, 270.1 GBq/mmol; AdreView) and 131I-MIBG (specific activity, 11.1–55.5 GBq/mmol) were purchased from GE Healthcare. 3H-labeled norepinephrine hydrochloride (3H-norepinephrine) was purchased from Perkin-Elmer. 18F-FDG was produced as previously described (20).
In Vitro Cell Uptake Assay for 131I-MIBG
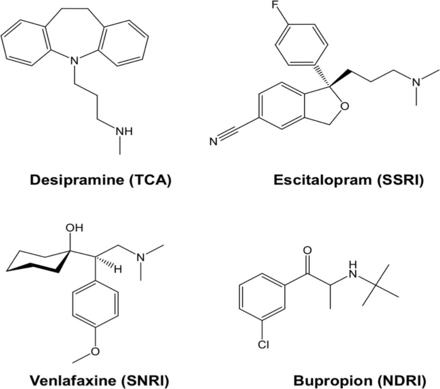
Human neuroblastoma SK-N-SH cells and desipramine were obtained from Sigma-Aldrich. Escitalopram was obtained from Lundbeck, venlafaxine from Betapharm, and bupropion from Neuraxpharm. An overview of the investigated antidepressants is given in Figure 1. SK-N-SH cells were grown in minimum essential medium with 2 mM l-glutamine and fetal bovine serum (10%). The cells were cultured in 75 cm2 flasks and later transferred to 12-well plates 1 d before the uptake assay. Increasing concentrations of antidepressants and 131I-MIBG (3.7 kBq/well) were added and incubated at 37°C for 1 h: desipramine (a TCA) at a dose range of 10 pM–10 μM, escitalopram (a SSRI) at 10 pM–100 μM, venlafaxine (a SNRI) at 10 pM–10 μM, and bupropion (a NDRI) at 10 pM–1 mM. After incubation, the cells were washed twice with ice-cold phosphate-buffered saline and solubilized with 0.1N NaOH. Radioactivity associated with the cells was measured using a γ-counter (FH 412; Frieseke and Höpfner), with the results expressed as counts per minute (cpm). 131I-MIBG cpm were normalized by dividing cpm of 18F-FDG. As a physiologic reference, the inhibition effect of desipramine and escitalopram on 3H-norepinephrine uptake was assessed in the same assay system. Assays were performed in medium containing inhibitors of catecholamine metabolism (100 μM pargyline and 20 μM pyrogallol). Because norepinephrine uptake peaks earlier in SK-N-SH cells than in 123I-MIBG, we adopted 30 min as the preferred tracer incubation time for the norepinephrine uptake assay. The radioactivity associated with the cells was measured using a liquid scintillation counter.
Structure of desipramine (a TCA), escitalopram (a SSRI), venlafaxine (a SNRI), and bupropion (a NDRI).
For both in vitro cell uptake studies, nonspecific uptake was determined in the presence of 10 μM desipramine, and specific cellular tracer uptake (%) was calculated by subtracting nonspecific uptake from total uptake. The inhibition rate for each antidepressant at each dose was calculated as a percentage of control. Dose–response curves were plotted to determine the half-maximal inhibitory concentration (IC50) and the percentage inhibition at the reported maximum concentration (Cmax) using ImageJ software (version 1.47; National Institutes of Health). Cmax was defined as the maximum concentration of a drug achieved after dosing as determined in clinical trials (21).
Animal Pretreatment
Female healthy New Zealand White rabbits (Charles River Laboratories) weighing 2,330 ± 825 g were used. The animal protocols were approved by the local Animal Care and Use Committee and conducted according to the Guide for the Care and Use of Laboratory Animals (22). Rabbits were assigned to one of the following pretreatments, which were administered via ear vein injection: desipramine, 1.5 mg/kg intravenously (n = 3); escitalopram, 2.5 (n = 5) or 15 (n = 4) mg/kg intravenously; or saline, administered intravenously as a control (n = 4).
123I-MIBG Scintigraphy
Ten minutes after pretreatment, 123I-MIBG (50 MBq) was injected intravenously. Scanning was performed 2.5 h afterward using a dual-head γ-camera (Symbia E; Siemens Healthcare) with a medium-energy collimator. For imaging, the animals were maintained under anesthesia with 2% isoflurane and placed prone on one detector head of the dual-head γ-camera. Chest scintigraphic images with ventral, anterior views of 10 min were acquired in 256 × 256 matrices with a zoom factor of 1.0 and an energy window set at 20% of the 159-keV 123I photopeak. Cardiac tracer accumulation was quantified according to the guidelines of the American Society of Nuclear Cardiology (23): heart-to-mediastinum ratio (HMR) was calculated by dividing the average count density of the manually drawn region of interest on the left ventricle by that of the mediastinal region of interest on anterior chest images.
Determination of Antidepressant Drug Concentration
Serum concentrations were determined using high-performance liquid chromatography, as previously described (24).
Statistical Analysis
All results are displayed as mean ± SD. The 2-tailed paired Student t test was used to compare differences between dependent groups, and the 2-tailed independent Student t test was used to compare differences between independent groups. A P value of less than 0.05 was assumed to be statistically significant. Statistical analysis was done with StatMate III (ATMS Co., Ltd.).
RESULTS
In Vitro Study
For desipramine, escitalopram, venlafaxine, and bupropion, the IC50 values on 131I-MIBG uptake were 11.9 nM, 7.5 μM, 4.92 μM, and 12.9 μM, respectively (Fig. 2); the inhibition rates of 131I-MIBG uptake at Cmax (as derived by clinical studies (21)) were 90.6%, 0.72%, 25.5%, and 11.7%, respectively; and the dose ranges were 33.8–237 nM, 17.9–94.9 nM, 122 nM–1.26 μM, and 75.1–526 nM, respectively. As a reference, the IC50 values of desipramine and escitalopram on 3H-norepinephrine uptake in the same assay system were 4.03 nM and 3.06 μM, respectively. The inhibition rates of 3H-norepinephrine uptake at Cmax were 99.2% for desipramine and 8.62% for escitalopram.
Dose–response curves from in vitro 131I-MIBG uptake assays using increasing concentrations of different antidepressants. Reported Cmax as derived by clinical studies (21) is displayed on each graph. Dotted lines indicate respective IC50.
In Vivo Study
Focal 123I-MIBG uptake indicating cardiac tracer accumulation was identified in all animals (HMR, 1.94 ± 0.22; Fig. 3). Pretreatment with the potent neuronal uptake 1–blocking agent desipramine, 1.5 mg/kg intravenously, diminished cardiac 123I-MIBG uptake (HMR, 1.23 ± 0.18; P < 0.001 vs. controls).
Results of in vivo rabbit 123I-MIBG studies comparing pretreatment with the SSRI escitalopram vs. the TCA desipramine. H/M ratio decreased significantly (*P < 0.01) after blockade of uptake-1 with desipramine (1.5 mg/kg) but not after increasing doses of escitalopram (2.5 and 15 mg/kg).
In the previous binding assay study, venlafaxine and bupropion showed considerable inhibitory effects on 123I-MIBG, whereas escitalopram demonstrated almost no inhibition of 123I-MIBG uptake at Cmax. Hence, to further definitely rule out an inhibitory potential, escitalopram was also tested in vivo: with a 2.5 mg/kg dose of escitalopram, no pharmacologic influence on cardiac 123I-MIBG uptake was observed (HMR, 2.01 ± 0.13). Even after the escitalopram dose was increased to 15 mg/kg, no substantial impact on 123I-MIBG uptake was seen (HMR, 2.05 ± 0.19; Fig. 3). Average serum concentrations of escitalopram after intravenous pretreatment with 2.5 and 15 mg/kg were 232 and 1,067 ng/mL, respectively (∼13- to 15-fold higher than standard clinical therapeutic concentrations of 15–80 ng/mL in humans (21)).
DISCUSSION
When interpreting the results of cardiac 123I-MIBG imaging, it is crucial that one recognize unintended effects of prescribed medications (6,7,25). Because of the large variety of drugs directly competing with 123I-MIBG for norepinephrine transporters, testing of these drugs should meet the following prerequisites: the drug should be widely prescribed, should follow the norepinephrine metabolic pathway, and should be investigated in a robust preclinical model that is clinically relevant and translatable. On the basis of these principles, we tested the 4 classes of antidepressants most prescribed for MDD treatment (10). These compounds are also known to interfere with NETs (6). The in vitro neuroblastoma cell lines that we used, as well as the rabbit model, are well-established methods for evaluating NET-related tracer mediation (4,26–28), and the sequence of investigating cardiac catecholamine analog tracers using SK-N-SH cells followed by a subsequent in vivo rabbit imaging study has been frequently described in the literature (28,29). As expected, pretreatment with desipramine led to a significant reduction of cardiac uptake, whereas venlafaxine and bupropion had moderate inhibitory effects. However, escitalopram demonstrated no in vitro interaction with 123I-MIBG (Fig. 2). In view of these initial findings, we also tested escitalopram on healthy rabbits to definitely rule out its inhibitory potential on 123I-MIBG uptake. The in vitro findings were corroborated, as no in vivo interaction with 123I-MIBG after escitalopram pretreatment was seen on scintigraphy (Fig. 3).
Tracer uptake via NETs forms the backbone for presynaptic sympathetic innervation imaging (30,31). Specificity for presynaptic uptake 1, as well as the mechanism of vesicular packaging inside the nerve terminals, differs from species to species and among available radiotracers (32). Even though smaller rodents such as rats might be easier to handle and more affordable, Rischpler et al. concluded that the rabbit heart would be a more attractive model for assessing sympathetic nerve conditions because specificity for uptake 1 seems higher in rabbits (33). Analogous to previous findings in rabbits, cardiac 123I-MIBG uptake via neural uptake 1 was reconfirmed with in vivo scintigraphy using the potent uptake 1–blocking agent desipramine (28). Hence, because the contribution of norepinephrine clearance to neural uptake 1 is also pronounced in the human heart (19), the rabbit heart offers an appealing platform for testing catecholamine analog tracers. The human neuroblastoma SK-N-SH cells that we used are also established as a robust model for NET-related tracer mediation (28): 3H-norepinephrine reflects the physiologic condition of norepinephrine (34), and its inhibition rate at Cmax using desipramine was 99.2% in the present study.
Escitalopram is classified as the most potent serotonin transporter–selective compound, a property predictive for antidepressant efficacy (15,35). However, the fact that escitalopram has moderate affinity for NETs (16,17) suggests potential interactions with 123I-MIBG uptake. In competition assays, escitalopram showed a serotonin transporter selectivity approximately 8,000–9,000 times higher than its NET selectivity (15). This finding is in line with our in vitro results demonstrating very low inhibition rates of 131I-MIBG uptake (0.72%) at Cmax. Additionally, the IC50 of escitalopram was significantly higher than its Cmax, suggesting that the potential maximum concentration may theoretically be reached long before the IC50 value is achieved (Fig. 2). In addition, our in vivo rabbit study found that escitalopram did not have a considerable effect on cardiac 123I-MIBG uptake, even in plasma blood concentrations considerably higher than clinical norms (21).
Because of the limited literature on the effect of antidepressants on 123I-MIBG pharmacokinetics, the level of evidence by which to judge the effects of SSRIs, SNRIs, TCAs, or NDRIs with a specific focus on cardiac studies is limited (6). Jacobson et al. primarily categorized patients on the basis of the potency (for NET interference) of their neuropsychiatric drugs: HMR ratios were significantly lower for patients receiving high-potency drugs than for those receiving low-potency drugs. However, a subanalysis for the influence of specific types of antidepressants on 123I-MIBG uptake could not be provided (25). Apart from that study, research has been conducted using platelets, pheochromocytoma cells, and neuroblastoma cells: analogous to our findings for the SSRI escitalopram, previous studies revealed that 123I-MIBG neuronal uptake is minimally affected by the SSRI fluvoxamine (6,36,37). The SNRI milnacipran moderately reduced 123I-MIBG uptake in the heart (18,38), similar to our binding assay results (inhibition rate at Cmax, 26% for the SNRI venlafaxine). A brain study using PET agents yielded comparable results for the occupancy of NETs in humans receiving milnacipran (39). In line with our findings for desipramine, Sisson et al. found a significant reduction in cardiac 123I-MIBG uptake 2 h after administration of the TCA imipramine to healthy volunteers (40). Recently, 123I-MIBG has been also introduced for evaluating Parkinson disease (41,42). Shimizu et al. investigated the usefulness of myocardial scintigraphy for differentiating dementia with Lewy bodies from Alzheimer disease, and any patients receiving the NDRI bupropion were excluded (43). To our knowledge, the present study provides the first evidence of reduced 123I-MIBG uptake in vitro (inhibition rate at Cmax, 12%). Hence, in a conservative approach, bupropion might be better discontinued before 123I-MIBG administration.
However, a limitation of our in vivo rabbit study is that hemodynamic data—including left ventricular function, heart rate, and blood pressure—were not recorded. Because of its anticholinergic properties, the TCA desipramine is known to have several effects on the heart, such as decreased blood pressure, electrophysiologic alterations, and myocardial contractility effects (44). On the other hand, SSRIs such as fluvoxamine and escitalopram seem to have only slight effects on heart rate, as their pharmacologic profile has almost no anticholinergic activity (44–46). Hence, given that TCAs have a potential influence on hemodynamics, one might speculate that desipramine may have caused a further decrease in cardiac 123I-MIBG uptake in the present in vivo study. Moreover, a potential gap between healthy conditions and cardiac impairment has to be addressed in future investigations. Although our goal was to provide a basic overview on how antidepressants influence cardiac 123I-MIBG uptake, an examination in heart-impaired animal models could also demonstrate whether detection of disease-based alterations is still robust under escitalopram administration.
Our findings suggest that TCA, SNRI, and NDRI therapy may interfere with NET binding in patients scheduled for 123I-MIBG cardiac imaging but that SSRI therapy can be continued in such patients without producing this confounding effect. Although further confirmation in humans is warranted, a well-designed trial might be difficult to achieve because of the absence of a verum arm, as a patient’s mental status might not tolerate withdrawal of therapy. Moreover, because patients are advised to taper rather than discontinue antidepressants (to reduce the risk of withdrawal symptoms (47)), determining when to obtain the 123I-MIBG scan would be difficult.
CONCLUSION
Our in vitro binding assay and in vivo rabbit study testing the antidepressant classes most prescribed for MDD found that, at therapeutic dosages, the SSRI escitalopram did not significantly interfere with neuronal 123I-MIBG uptake whereas the other classes of antidepressants did. Further assessment to reveal the potential interference of neuropsychiatric medications on cardiac 123I-MIBG uptake is warranted.
DISCLOSURE
This work was supported by the Competence Network of Heart Failure, funded by the Integrated Research and Treatment Center (IFB) of the Federal Ministry of Education and Research (BMBF) and the German Research Council (DFG grant HI 1789/3-3). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 701983. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Mar. 1, 2018.
- © 2018 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 28, 2017.
- Accepted for publication February 12, 2018.