Abstract
A potential milestone in personalized nuclear medicine is theranostics of metastatic castration-resistant prostate cancer (mCRPC) based on molecular imaging using PET/CT with 68Ga-labeled prostate-specific membrane antigen (PSMA) ligands and molecular radiotherapy using PSMA-targeted radioligand therapy (PRLT) with 177Lu-PSMA ligands. 68Ga-PSMA PET/CT enables accurate detection of mCRPC lesions with high diagnostic sensitivity and specificity and provides quantitative and reproducible data that can be used to select patients for PRLT and therapeutic monitoring. Our comprehensive experience over the last 3 years using different radioligands indicates that PRLT is highly effective for the treatment of mCRPC, even in advanced cases, and potentially lends a significant benefit to overall and progression-free survival. Additionally, significant improvement in clinical symptoms and excellent palliation of pain can be achieved.
Metastatic castration-resistant prostate cancer (mCRPC), defined as disease progression despite medical or surgical castration, develops in 10%–20% of prostate cancer patients (1). Most CRPC patients have metastases at diagnosis or develop widespread disease during the first 2 years of follow-up. First-line chemotherapeutic agents such as docetaxel, and second-line chemotherapy with cabazitaxel, are often toxic and prolong life by only a few months (2). Postchemotherapy androgen deprivation therapy with abiraterone acetate and enzalutamide has been shown to prolong overall survival by only 3.9 and 4.8 mo, respectively, compared with placebo. Likewise, the overall survival benefit, compared with placebo, is only 3.6 mo for 223Ra-chloride (3–5), which targets only osteoblastic lesions and does not treat nodal or visceral metastases.
PET/CT using 68Ga-labeled ligands targeting the prostate-specific membrane antigen (PSMA) is a sensitive diagnostic modality, especially in poorly differentiated prostate cancer. Its sensitivity in a recent retrospective series by Eiber et al. improved with rising prostate-specific antigen (PSA) velocity, reaching 100% for a PSA velocity of 5 ng/mL/y or more and a Gleason score of 8 or more (6). PSMA-targeted radioligand therapy (PRLT) involves selective binding of a radioligand to PSMA overexpressed in mCRPC in order to increase tumor dose and spare normal tissue (7,8). Internalization and retention within the tumor cell are essential mechanisms for the cell-killing effect of this radiation therapy (also called endoradiotherapy), which has the advantage of selectively targeting multiple metastases (9).
Theranostics using PET/CT as an in vivo companion diagnostic for decision making and monitoring of radiolabeled peptide therapy or PRLT is an integral part of personalized nuclear medicine (10,11). PSMA-specific PET/CT with 68Ga, and PRLT with therapeutic radionuclides such as 177Lu, offer a new and unique theranostic possibility by using similar ligands for both diagnosis and therapy. 68Ga-PSMA PET/CT is pivotal in selecting mCRPC patients with strong PSMA expression, identifying responders, and personalizing PRLT regimens (12). There is ample evidence of the utility of PET/CT in assessing response, such as with 18F-FDG after radioimmunotherapy of non-Hodgkin lymphoma and with 68Ga-somatostatin analogs after peptide receptor radionuclide therapy of neuroendocrine tumors (13,14). Similarly, in PRLT, 68Ga-PSMA PET/CT could play an important role in determining prognosis, monitoring therapy, and following up over the long term.
J591 was the first humanized monoclonal antibody targeting the extracellular domain of PSMA (14). Radiolabeled J591 accurately targets bone and soft-tissue prostate cancer metastases for diagnosis or therapy (15). Radioimmunotherapy using 177Lu-J591 demonstrated antitumor activity and acceptable tolerability in a phase 1 clinical trial (16). To our knowledge, we were the first to use the genetically engineered PSMA minibody IAb2-M-DOTA labeled with 111In for imaging and dosimetry in patients. The 111In-labeled compound showed significant uptake in metastatic lesions (17), but hepatic uptake was high when 1 mg was used. Increasing the amount of minibody achieved lower hepatic uptake in accordance with results obtained after administration of the intact J591 parent antibody (15,17).
The research group at Johns Hopkins, in 2002, was the first to implement imaging with a small molecule that targets PSMA (18). The related compounds 123I-MIP-1072 and 123I-MIP-1095, also bearing a urea-based scaffold, have been used to detect metastatic prostate cancer with SPECT (19). These small molecules demonstrated a potential for therapy when labeled with 131I (20). On the basis of biodistribution and dose calculations for the PSMA-targeted small molecule 124I-MIP-1095, the Heidelberg group at the German Cancer Research Center developed a therapy with the analog 131I-MIP-1095 that enabled targeted delivery of unprecedented doses to the malignant tissue. The involved lymph node and bone metastases were exposed to mean absorbed doses of more than 300 Gy. However, there was significant toxicity to the salivary glands, with documented xerostomia and mucositis. No renal dysfunction occurred, but thrombocytopenia was noticed (20).
We report here our experience at Zentralklinik Bad Berka since we started, in April 2013, using PRLT with a 177Lu-labeled small molecule targeting PSMA.
INDICATION FOR PRLT
PRLT using 177Lu-labeled PSMA ligands is a novel and highly targeted systemic therapy for progressive mCRPC, especially after exhaustion of available conventional therapies such as androgen deprivation and treatment with abiraterone and enzalutamide, as well as taxane-based chemotherapy (Table 1). Distant metastases with high PSMA expression confirmed on pretherapy 68Ga-PSMA PET/CT, and progressive disease despite extensive previous treatments, are currently essential inclusion criteria as stated in the 2016 consensus recommendations of the German Society of Nuclear Medicine (21). An already decreased bone marrow reserve before PRLT—due to prior chemotherapy or 223Ra-therapy of skeletal metastases or to replacement of normal bone marrow by advanced metastatic disease—may predispose to increased bone marrow toxicity (Table 2). PRLT should be performed after correction of anemia by transfusion of red blood cells, which is also imperative to potentiate the tumoricidal effect of molecular radiotherapy by ensuring an oxygen concentration adequate for the formation of free radicals (22).
Current Prerequisites for PRLT
Possible Risk Factors to Consider Before PRLT
THE BAD BERKA EXPERIENCE
177Lu-PSMA-TUM1
177Lu-labeled DOTAGA-FFK(Sub-KuE)—developed by the research group at Technical University Munich and given the term 177Lu-PSMA-TUM1—led to a significant reduction in metastatic tumor load in 6 mCRPC patients who underwent PRLT using 4.2–8.6 GBq of this compound (23). 68Ga-PSMA PET/CT was performed on all patients 1–5 d before therapy and during follow-up. High PSMA expression extensively in bone and lymph nodes (n = 4), as well as in lung metastases (n = 1) and in residual or locally recurrent prostate cancer (n = 2), was confirmed by baseline PET/CT and by 177Lu-PSMA-TUM1 uptake on posttherapy planar and SPECT/CT images. PSA fell by more than 30% in 4 of the 6 patients and by more than 50% in 2 of the 6. Follow-up PET/CT of 3 patients after a single course of PRLT demonstrated partial remission in 2, with an SUVmax decrease by 51% and 89% in the target lesion and disappearance of many previously noted PSMA-positive metastases. There was progression of skeletal metastases in 1 patient. 177Lu-PSMA-TUM1 exhibited high tumor uptake, fast renal washout, and rapid blood clearance, resulting in a favorable dosimetry. The following organ and tumor doses were calculated: whole body, 0.02 ± 0.01 mGy/MBq; kidneys, 0.33 ± 0.15 mGy/MBq; and tumors, 0.14–5.5 mGy/MBq. Clearance from blood was rapid, with a half-life of up to 42 h. The treatment was tolerated well by all patients, without any significant adverse effects or alterations in any laboratory parameters.
177Lu-PSMA-I&T
Weineisen et al. focused on further enhancing the affinity and improving the in vivo properties of TUM1 derivatives by increasing the lipophilic interaction of the tracer with PSMA (9). Thus, DOTAGA-(I-y)fk(Sub-KuE), given the term 177Lu-PSMA-I&T (for imaging and therapy), was developed and evaluated in detail. Our group analyzed the safety and efficacy of 177Lu-PSMA-I&T in 56 patients with mCRPC and found a high objective response rate (7) that resulted in a long progression-free and overall survival (with potential survival benefit) with minimal toxicity.
177Lu-PSMA-617
The small molecule PSMA-617 was demonstrated by recent studies to be safe for PRLT and to have a low toxicity profile, in line with our own experience (24–26).
The different 177Lu-labeled ligands (PSMA-TUM1, PSMA-I&T, and PSMA-617) used for PRLT yielded similar clinical results, considering efficacy and toxicity profiles.
177Lu-PSMA-SRV171
In 2015 we performed the first 68Ga-based PET/CT imaging in patients of mCRPC using a novel PSMA ligand, SRV171 (Fig. 1), which is a potential addition to the existing armamentarium for PRLT when labeled with 177Lu.
First-in-human 68Ga-SRV171 PSMA PET/CT imaging demonstrating extensive and excellent tracer uptake in soft-tissue (lymph node) and skeletal metastases (arrows). (A) PET maximum-intensity-projection image. (B) Axial PET/CT image of thorax. (C) Axial PET/CT image of abdomen.
PRACTICAL PROCEDURE
The radiopharmaceutical is administered over 5 min with an infusion pump dedicated for radionuclide therapy. In our experience (>400 PRLT administrations), the monitored vital parameters (temperature, pulse, and blood pressure) did not significantly change during or after therapy. Hence we suggest that documentation of these vital parameters once before and once after therapy is sufficient. The patients must be well-hydrated, should be encouraged to drink at least 1.5–2 L of fluid, and should receive a 1,000-mL infusion of saline containing 20–40 mg of furosemide after therapy. In contrast to peptide receptor radionuclide therapy with somatostatin analogs, no special nephroprotective measures are required, as the renal absorbed doses were unaffected by whether nephroprotection was used (unpublished data).
DOSIMETRY COMPARISON BETWEEN 177LU-PSMA-I&T AND 177LU-PSMA-617
A comparative intrapatient (i.e., for different PRLT courses) and interpatient dosimetry analysis was performed for 177Lu-PSMA-I&T and 177Lu-PSMA-617 (Fig. 2; Table 3). However, a direct head-to-head comparison has some limitations: first, response to therapy and differential tumor load in a particular patient may influence dosimetry after 2 different PRLT courses; second, an interpatient comparison cannot yield completely accurate results, since the kinetics of a certain ligand depend on conditions such as renal function and tumor load. In our experience, the ligands 177Lu-PSMA-I&T and 177Lu-PSMA-617 did not significantly differ in response and toxicity profiles.
Biodistribution and dosimetry results for normal organs in patients treated with different PSMA radioligands (median uptake in percentage injected activity [%IA]). (A) Kinetics. (B) Effective half-life in hours. (C) Mean absorbed dose in mGy/MBq (n = 38 for 177Lu-PSMA-I&T and n = 19 for 177Lu-PSMA-617).
Absorbed-Dose Constraints and Maximum Activity and Cycles to Reach Them
Whole Body
We observed a higher whole-body uptake for 177Lu-PSMA-617 at all scan time-points. The time–activity curves demonstrated a biexponential function, that is, an initial rapid decline followed by a second slower decline. The half-lives were shorter for 177Lu-PSMA-I&T than for 177Lu-PSMA-617, which showed a slower washout. The whole-body dose was moderately higher for 177Lu-PSMA-617 because of significantly higher uptake at 20 h after injection and a longer effective half-life.
Kidneys
Renal uptake was slightly higher for 177Lu-PSMA-I&T. For both ligands, renal uptake declined rapidly between the first scan and 3 h after injection, followed by a slower washout with comparable half-lives. The resulting renal dose was slightly higher for 177Lu-PSMA-I&T than for 177Lu-PSMA-617.
Parotid Glands
In the parotid glands, both ligands demonstrated an initial accumulation of activity until 3 h after injection followed by an exponential washout. 177Lu-PSMA-617 showed higher uptake values and longer half-lives. However, the resulting mean absorbed doses of the different ligands were comparable.
Lacrimal Glands
Higher uptakes and longer half-lives were observed for 177Lu-PSMA-617, in contrast to 177Lu-PSMA-I&T, resulting in higher mean absorbed doses to lacrimal glands using 177Lu-PSMA-617.
Red Marrow
For red marrow, the longest half-life was 94 h; the resulting mean absorbed dose was low, with a mean of 0.03 mGy/MBq (similar for the 2 ligands). In view of these results concerning the mean absorbed dose to red marrow with a dose limit of 2 Gy, the patients could theoretically be treated using injected activities of up to 200 GBq.
Metastases
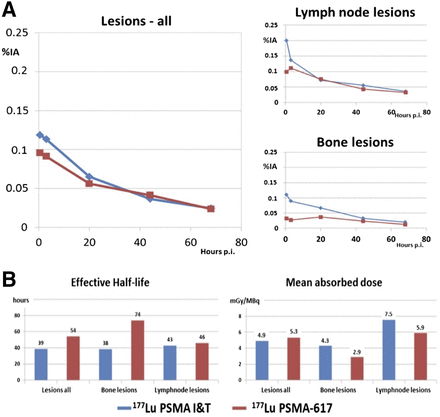
We comparatively analyzed 66 metastases (bone, lymph node, liver, lung, and other) in patients treated with 177Lu PSMA-I&T and 27 metastases (bone and lymph node) in patients treated with 177Lu-PSMA-617 (Fig. 3). A comparable uptake of both ligands was seen at 20 h after injection and later, but there were differences early after infusion (0.5 h after injection). 177Lu-PSMA-I&T exhibited a higher initial uptake. After fitting of all time–activity curves to monoexponential functions from 20 h after injection, a longer half-life was revealed for PSMA-617. The differences in resulting absorbed tumor doses were, however, statistically insignificant. The half-life for 177Lu-PSMA-617 was longer in bone lesions. Despite the relatively shorter half-life of 177Lu-PSMA-I&T as compared with 177Lu-PSMA-617, the dose estimations revealed that the highest doses for lymph node lesions occurred when 177Lu-PSMA-I&T was used. For both ligands, the mean absorbed dose was higher for lymph node metastases than for bone metastases.
(A) Kinetics of tumor lesions and comparative results using 177Lu-PSMA-I&T (blue) and 177Lu-PSMA-617 (red) for all lesions, lymph node metastases, and bone metastases. (B) Dosimetric results for different metastases. %IA = percentage injected activity; p.i. = after injection.
PRINCIPLES OF PRLT
PRLT is based on the principle of personalized medicine, in which number of cycles and amount of administered radioactivity depend on various factors (Table 4). Because bone is the most common site of metastases, a patient with predominantly bone lesions may require application of a relatively higher radioactivity than does a patient with predominantly lymph node lesions. However, although low, the risk of hematotoxicity in a patient with disseminated bone and bone marrow involvement must be kept in mind.
Factors to Consider in Determining Therapeutic Activity and Number of PRLT Cycles
The aim of the first 3 therapy cycles is to achieve remission or stabilization of disease (Fig. 4). Nearly all mCRPC patients who initially respond to PRLT will relapse. In our experience, PRLT can be safely repeated, inducing regression of the disease again (Fig. 5) (27). The maximum tolerable dose of PRLT has not yet been systematically studied. However, frequent therapy cycles (maximum of 8 in our experience so far) over a longer time are feasible for personalized management of the disease. The highest administered activity in a single cycle was 9.7 GBq of 177Lu-PSMA, and this level of activity had no serious adverse effects.
Complete remission of disease and 100% decline in serum PSA, sustained for over 4 mo after PRLT. (A) Numerous 68Ga-PSMA–avid skeletal metastases on PET/CT maximum-intensity-projection image before PRLT. (B–D) Excellent uptake on 177Lu images during first treatment (B, whole-body anterior image 20 h after injection) (C and D, SPECT/CT images 41 h after injection). (E–G) Significantly reduced uptake on images after second PRLT 2 mo later (E, whole-body anterior image 20 h after injection) (F and G, SPECT/CT images 45 h after injection). (H) Complete resolution of metastases on 68Ga-PSMA PET/CT image 4 mo after initiation of PRLT.
(A) Serial 177Lu-PSMA whole-body images obtained around 20 h after injection during (from left to right) first to seventh PRLT cycles. (B) PSA course during the 7 PRLT cycles. Images during first 4 treatments (in posterior view to better demonstrate the findings) show excellent response of multiple lymph nodes and some bone metastases to therapy, whereas images during 5th and 6th treatments (anterior view) show progressive disease with new extensive bone and bone marrow metastases, and image during 7th treatment (anterior view), in March 2016, shows partial remission of bone metastases and significant decrease in uptake that correlates well with significant PSA fall after sixth PRLT cycle, as seen in panel B.
COMPREHENSIVE RESULTS OVER 3 YEARS
Between April 2013 and April 2016, 119 mCRPC patients (median age, 71 ± 7 y; mean Gleason score, 8 ± 1) underwent 300 cycles of PRLT. The patients received 1–7 treatments, applying a median activity of 6.0 GBq/cycle (range, 2–9.7 GBq). Bone metastases were present in 96 patients (80.7%), whereas 84 (70.6%) presented with lymph node metastases and 19 (16.0%) with visceral metastases. Notably, 24 patients (20.2%) had bone metastases only but were not candidates for 223Ra therapy. Lymph node lesions involving more than 2 nodal regions predominated in 19 patients (16.0%), who had no evidence of skeletal metastases on imaging.
Determination of serum PSA level after at least one course of PRLT in 80 patients revealed a reduction of PSA level in 61 patients (76.3%), by more than 50% in 46 (57.5%) and more than 80% in 22 (27.5%) (Fig. 6A). There was a biochemical complete remission in 2 patients (2.5%), who had no detectable PSA after PRLT.
(A) Best percentage changes in baseline serum PSA level in 80 patients during follow-up period. (B) Best percentage changes in baseline SUVmax in 57 patients during follow-up period.
Pain lessened and quality of life improved significantly in symptomatic patients. For example, a dramatic reduction in bone pain was observed in patients who had been wheel chair–bound before PRLT but could walk without support after PRLT.
In general, the patients tolerated the treatment well and experienced no severe acute or long-term (observation period, 34 mo) side effects. The most common adverse effect was mild fatigue lasting a few days after therapy. In addition, 5 patients (4.2%) reported mild dryness of the mouth, most cases of which were reversible. Because of a potentially high radiation dose to the salivary glands, the effect on function can be reproducibly assessed using dynamic salivary gland scintigraphy with 99mTc-pertechnetate before and after PRLT.
Notably, there was no diarrhea despite significant intestinal uptake, in contrast to 223Ra therapy (5). On the contrary, the Heidelberg group reported obstipation as a side effect, which we believe may have been due mainly to the intake of opioid analgesics, as it was not frequently reported in our patient cohort (25). There was no evidence of renal toxicity after PRLT; that is, no significant change was observed in serum creatinine, creatinine clearance (obtained by the Cockcroft–Gault formula), or tubular extraction rate as determined by 99mTc-mercaptoacetyltriglycine (MAG3) renal scintigraphy. In the initial experience with 177Lu-PSMA-I&T, no grade 3 or 4 hematologic toxicity was found. However, in the extended patient group reported here, 4 patients (3.4%) had grade 3 or 4 hematologic toxicity. These patients had been heavily pretreated with chemotherapy or 223Ra-therapy of bone metastases and had an already-compromised bone marrow reserve before PRLT. Diffuse bone marrow involvement in combination with previous chemotherapy or 223Ra-therapy represents a risk for the development of hematotoxicity. In such cases, therapy with PSMA ligands labeled with α-emitters could be beneficial for a highly targeted treatment of metastases (28).
68Ga-PSMA PET/CT is a sensitive and specific modality for the detection of both soft-tissue and bone metastases in prostate cancer (29). The response to therapy was assessed using RECIST 1.1 (on contrast-enhanced CT) and molecular response criteria—that is, by determining the change in SUV using the criteria of the European Organization for Research and Treatment of Cancer. 68Ga-PSMA PET/CT after at least two courses of PRLT was analyzed in 58 patients (Fig. 6B). The best molecular response (according to the criteria of the European Organization for Research and Treatment of Cancer) was complete remission in 5 patients (8.6%). Partial remission was documented in 12 (20.7%), and stable disease in 23 (39.7%). Thus, disease control (reversal of progression [stability or remission of disease]) was achieved in 40 patients (69.0%), and mCRPC continued to progress in 18 (31.0%).
Seventeen patients with extensive metastases (>20 bone or lymph node metastases) presented with a PSA level of less than 10 ng/mL, and their PSA value did not correlate with disease burden. This finding may be explained by dedifferentiation of metastases, which therefore manifest with a low serum PSA but a high PSMA-specific uptake. In 6 of these patients, the less than 25% rise in PSA level was deemed insignificant in light of the stable disease course on PET/CT. On the other hand, 2 patients demonstrated only a mild decrease in serum PSA but a significant reduction in uptake on 68Ga-PSMA PET/CT (partial remission based on molecular response criteria); 1 of these 2 patients also exhibited disease progression based on RECIST 1.1. Both of these patients presented with PSA values of less than 1 (0.77 and 0.05 ng/mL, respectively). In the patient with progressive disease by RECIST, there was less than a 25% PSA increase; however, this patient also presented with a low PSA level, which did not correspond with the high disease burden.
In general, we found that lymph node metastases of CRPC responded better to PRLT than bone metastases. This finding may be explained by a higher and more uniform radiation dose absorbed by lymph node metastases, which generally exhibit a higher uptake (SUV) on 68Ga-PSMA PET/CT than do bone lesions. In addition, the biologic differences in radiation sensitivity might be an influencing factor. 68Ga-PSMA PET/CT was superior to CT alone in assessing the response of skeletal metastases, as the actual size of osteoblastic metastases is difficult to measure and change in size is difficult to appreciate on CT alone. Especially in lymph node metastases, 68Ga-PSMA PET/CT detects response at an earlier stage than does CT alone (i.e., molecular response precedes morphologic changes).
In refractory advanced disease, a combination of therapies is often more effective. Six cycles of docetaxel at the beginning of androgen deprivation therapy for mCRPC resulted in a 13.6-mo longer median overall survival than that with androgen deprivation therapy alone (30). Androgen deprivation therapy may have a synergistic effect with PRLT, as PSMA genes are suppressed by androgens and hence PSMA is upregulated by androgen deprivation in mCRPC (31). Therefore, we recommend continuation of treatment with luteinizing hormone–releasing hormone analogs during PRLT.
Our patient cohort was quite heterogeneous, having a generally high Gleason score and disease burden—including diffuse bone marrow and visceral involvement—and progressive disease despite extensive pretreatment. Overall, high response rates could be achieved with minimal toxicity despite these negative prognostic factors. The survival data were analyzed in 104 patients (Fig. 7). Over a follow-up period of 34 mo (median, 19 mo), 26 patients died (25%). The median overall survival has yet to be reached. Progression-free survival from the commencement of therapy was estimated to be 10.7 mo. The already-compromised bone marrow function due to disseminated bone marrow involvement or prior myelosuppressive therapies (e.g., chemotherapy or 223Ra-therapy) limits the application of a higher radioactivity. This limitation might explain a poorer therapeutic response in such patients and the relatively shorter calculated PFS therefore resulting for the entire patient cohort. Potential further treatment cycles could be considered in these patients, taking into account the persistently high PSMA expression of metastases in combination with newer agents such as checkpoint inhibitors (32). The ideal patient for PRLT could possibly be one receiving it before chemotherapy (or maybe in combination with chemotherapy) with good baseline bone marrow function and a good baseline performance status. Therefore, PRLT could potentially be considered at an earlier stage of the disease. The use of PRLT in sequence or in combination with chemotherapy, newer agents (abiraterone, enzalutamide), immune checkpoint inhibitors, and administration of higher radioactivity at shorter intervals should be addressed in future studies.
Kaplan–Meier curves showing overall (A) and progression-free (B) survival, according to RECIST 1.1, in 104 patients (observation period, 34 mo).
CONCLUSION
PRLT using 177Lu-labeled PSMA ligands is highly effective for the treatment of mCRPC, even in advanced cases. In addition, significant improvement in clinical symptoms and excellent palliation can be achieved with low or minimal toxicity. Further prospective randomized clinical trials for head-to-head comparison with other treatment modalities are warranted to allow for an unbiased evaluation of survival data and consideration of PRLT earlier in the line of management for mCRPC.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We would like to express our sincere gratitude to Michael Hofman and Martin Pomper for their critical reading of the manuscript and valuable discussions. We also thank our physician colleagues, the nursing staff, and the nuclear medicine technologists for their support, as well as Sangeeta Ray for the collaboration.
- © 2016 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication July 3, 2016.
- Accepted for publication August 22, 2016.