Abstract
The aim of this study was to assess the safety, tolerability, and effects on renal function as well as therapeutic efficacy of prostate-specific membrane antigen (PSMA)–targeted radioligand therapy (PRLT) using 177Lu-labeled PSMA-617 in patients with metastatic castration-resistant prostate cancer and a single functioning kidney before PRLT. Methods: Sixteen patients (aged 53–78 y; mean age, 64.7 ± 6.5 y) with a single functioning kidney received PRLT with 177Lu-PSMA-617 between March 2015 and October 2018. All parameters of renal function (serum creatinine, blood urea nitrogen, and electrolytes) were prospectively documented in a structured database and analyzed before each PRLT cycle and in follow-up. Renal function was further quantified by measuring tubular extraction rate (TER) using 99mTc-mercaptoacetyltriglycine renal scintigraphy. Treatment-related adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Kaplan–Meier analysis was performed to obtain the progression-free survival and overall survival. Results: The median administered activity was 22.1 GBq (range, 15.4–33.8 GBq). The calculated absorbed radiation dose to the kidney per cycle was 5.3 ± 2.1 Gy (0.81 ± 0.32 Gy/GBq). Renal function was already impaired at baseline in 43.7% of patients, including CTCAE grade 1 renal impairment in 25.0% and CTCAE grade 2 in 18.8%. Grade 1 and 2 renal impairment, respectively, were present in 37.5% and 6.3% of the patients after the first PRLT cycle and in 31.3% and 12.5% after the second cycle. No CTCAE grade 3 or 4 nephrotoxicity was observed during or after treatment. There was no significant change in either TER or the ratio of TER to lower-limit TER after the last cycle of treatment (P > 0.05). The median PFS was 8.1 mo based on both the criteria of the European Organization for Research and Treatment of Cancer and RECIST. The median overall survival has yet to be reached with a median follow-up time of 19.3 mo (range, 5.8–45.3 mo). Conclusion: In patients with a single functioning kidney, 177Lu-PSMA-617 PRLT is feasible, seems to be effective, and is well tolerated, without any signs of acute or subacute nephrotoxicity during a mean follow-up of nearly 2 y (and up to 45.3 mo). Further long-term follow-up of this special patient group is warranted.
Prostate cancer is the second most common cancer and the fifth leading cause of death in men worldwide, with an estimated 1.3 million new cases and 359,000 associated deaths worldwide in 2018 (1). Castration-resistant prostate cancer is defined by progression occurring in the setting of castration levels of testosterone (2,3). Metastatic castration-resistant prostate cancer (mCRPC) is associated with a poor prognosis (4,5). Almost all patients with androgen-sensitive metastatic disease respond to androgen deprivation therapy but tend to progress to castration-resistant disease at some point in time (6,7), which, invariably, is the main cause of death in prostate cancer patients (8).
Prostate-specific membrane antigen (PSMA), known as folate hydrolase I or glutamate carboxypeptidase II, is a transmembrane glycoprotein primarily expressed in normal human prostate epithelium (9), as well as (at lower levels) in the small intestine, proximal renal tubules, and salivary glands (10). PSMA is highly expressed predominantly in advanced-stage, poorly differentiated, metastatic, hormone-refractory carcinomas, such as mCRPC, which presents a promising target for molecular imaging and molecular radiotherapy using radiolabeled small molecules. In recent years, there has been enormous progress in the use of PSMA-targeted small molecules, linked by a chelator to positron emitters for diagnosis as well as α-or β-particle–emitting isotopes such as 177Lu, 90Y, or 225Ac for targeted radionuclide therapy (11–14). Because of specific binding and internalization, the therapeutic radiolabeled PSMA small molecules accumulate in cancer tissues and lead to internal irradiation of the tumor cells.
177Lu is a medium-energy β-particle emitter with a half-life of 6.65 d, as well as a low-energy γ-emitter, which allows delayed imaging, therapy (theranostics), and dosimetry with the same radioisotope. Molecular radiotherapy using 177Lu-labeled PSMA ligands has demonstrated promising and encouraging results in mCRPC, even after exhaustion of all available conventional therapies, with relief of pain from bone metastases, decline of prostate-specific antigen, and objective remission with significant survival benefit (15–17). The latest studies in patients show that the treatment with 177Lu-labeled PSMA small molecules is well tolerated with no or only mild side effects, mainly related to bone marrow and salivary glands (5,18,19).
However, high posttherapy uptake in kidneys has been reported as a concern for nephrotoxicity. Because PSMA is specifically expressed by the renal tubules, the mean absorbed radiation dose to the kidneys is potentially significant (16). Therefore, PSMA radioligand therapy (PRLT) using 177Lu-PSMA in patients with already known renal insufficiency or a single functioning kidney raises concern about tolerability and potential nephrotoxicity. To our knowledge, this concern has not yet been addressed in clinical practice. The aim of this study was to assess the safety, tolerability, and effects on renal function, as well as the therapeutic efficacy, of PRLT using 177Lu-PSMA-617 in patients with mCRPC and a single functioning kidney.
MATERIALS AND METHODS
Patients
Sixteen patients (aged 53–78 y; mean age, 64.7 ± 6.5 y) with mCRPC and a single functioning kidney met the eligibility criteria (histologically proven prostate adenocarcinoma with nonresectable metastases, tumor progression under androgen deprivation therapy or other treatments, PSMA expression of metastases on PSMA PET/CT) to be included in this study. All patients had received androgen deprivation therapy, 9 (56.3%) had undergone radical prostatectomy, and 6 (37.5%) had received external-beam radiation therapy. Twelve (75.0%) patients had prior chemotherapy, including 7 patients receiving docetaxel, 3 receiving cabazitaxel, and 2 receiving both docetaxel anhydrous (Taxotere; Aventis Pharmaceutical, Inc.) and cabazitaxel. The demographics of the patients at baseline are given in Tables 1 and 2. All patients had extensive, PSMA-positive metastases confirmed on 68Ga-PSMA PET/CT. The activity of 177Lu-PSMA-617 administered and the number and interval of cycles were personalized and based on multiple factors such as uptake on 68Ga-PSMA PET/CT before therapy, renal function, hematologic status, previous treatments, and Karnofsky performance score. PET/CT (Biograph mCT Flow 64; Siemens Medical Solutions AG) was performed 60–80 min after intravenous administration of 68Ga-PSMA. All patients received 20 mg of furosemide intravenously to accelerate renal tracer excretion. Molecular and morphologic responses were evaluated in accordance with the criteria of the European Organization for Research and Treatment of Cancer (EORTC) (20–22) and RECIST 1.1 (23), respectively. Serum prostate-specific antigen response was documented at least monthly. 68Ga- and 177Lu-PSMA were administered in compliance with the German Medicinal Products Act (section 13, subsection 2b), the 1964 Declaration of Helsinki, and the responsible regulatory body (Government of Thuringia). All patients received 177Lu-PSMA PRLT under the compassionate-use clause of the German Medicinal Products Act (24). The decision to perform PSMA PRLT was based on the opinion of the referring urologists and oncologists after exhaustion of other therapeutic options. The study was performed in accordance with the regulations of the German Federal Agency for Radiation Protection. This retrospective study was performed in accordance with German regulations (Federal Agency for Radiation Protection) concerning radiation safety and was approved by the local ethics committee (Bad Berka, Germany). Written informed consent was obtained from all patients.
Patient Characteristics
Characteristics and Renal Toxicity Profile of Patients with mCRPC and Single Functioning Kidney (n = 16)
Treatment Regimen
177Lu labeling of the PSMA-617 ligand was performed in our radiopharmacy department in accordance with good medical practice using previously published methods (5,13). In brief, the PSMA ligand was incubated with the required radioactivity of 177Lu-Cl3 at 90°C for 30 min in sodium acetate buffer (0.4 M, pH 5.5). To this buffer, 5–10 mg of gentisic acid were added to prevent radiolysis. Quality control parameters were monitored (radiochemical purity, radiochemical identity, pH, ethanol content, endotoxin content, and proof of sterility). Radiochemical purity was more than 97% in all cases (in most labeling procedures, >99%). 177Lu-PSMA-617 was administered by slow intravenous injection over 10–15 min with a dedicated second infusion pump system for radionuclide therapy. Whole-body scintigraphy after therapy was performed with a SPIRIT DH-V dual-head γ-camera (Mediso Medical Imaging Systems) using a medium-energy general-purpose collimator, a 15% energy window with a peak at 208 keV, and a scan speed of 15 cm/min. Whole-body scintigraphy was acquired at 5 time points from 0.5 to 118 h after injection. SPECT/CT imaging was performed between 45 and 118 h after injection.
Dosimetry was performed in accordance with our previously described protocol (25). Time-dependent activity in kidney, other normal organs, and tumors was determined by drawing regions of interest on serial 177Lu-PSMA whole-body scans after therapy. The time–activity curves of source regions were fitted to exponential functions of the first or second order to determine the time-integrated activity. The mean absorbed doses were estimated with OLINDA/EXM software.
Toxicity Assessment
Details were prospectively documented in a structured database (comprising over 250 items per patient). The parameters analyzed included patient characteristics, clinical reports during the treatment, and renal toxicity profile before each PRLT cycle and in follow-up (restaging was performed regularly until death). Laboratory data including serum creatinine, blood urea nitrogen, electrolytes, blood, renal panel, liver panel, and others were evaluated. Acute or subacute and gradual losses of renal function were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Renal function was further quantified by measuring the tubular extraction rate (TER) using 99mTc-mercaptoacetyltriglycine renal scintigraphy. TER was derived from the measured 99mTc radioactivity concentrations in plasma samples corrected for body surface area (26). Age-adjusted mean normal values (TERNorm) were computed according to the following formula: TERNorm = [435 – (3.03 × age (y))] mL/min/1.73 m2. The lower limit of the reference range (TERLoLi) was 70% of TERNorm (27). The TER/TERLoLi ratio was calculated to correct for the normal decrease in renal function with age during the course of treatment for the individual patient, and to make data from individuals of different ages comparable.
Response Assessment
Treatment response was evaluated by structural imaging (CT or MRI) according to RECIST 1.1 as well as by molecular imaging criteria (EORTC) and by measuring prostate-specific antigen. Survival of the patients was also determined.
Statistical Methods
Continuous variables were summarized as mean ± SD. The rates of adverse events at baseline and at the end of 1–3 cycles of treatment were compared. Nonparametric sign tests were used for determination of the significance of differences between renal parameters before and after therapy. Differences among groups were compared with 1-way ANOVA. Kaplan–Meier survival analysis was performed to calculate the progression-free survival and overall survival defined as the time from the start of PRLT. The correlation between quantitative parameters was evaluated by the Pearson correlation coefficient for data with a normal distribution or the Spearman correlation coefficient for data with a skewed distribution. P values of less than or equal to 0.05 were considered significant.
RESULTS
In total, 54 PRLT cycles were administered. The median administered activity was 22.1 GBq (range, 15.4–33.8 GBq) in 2–6 cycles. Treatment cycles and cumulative radioactivity are summarized in Table 3. The median duration of follow-up from the start of therapy was 19.3 mo (range, 5.8–45.3 mo).
Treatment Cycles and Accumulative Radioactivity for 177Lu-PSMA-617 PRLT
Safety and Clinical Assessment
No life-threatening or disabling adverse events were observed after PRLT, and there were no clinically significant changes on physical examination. Short-lasting intensification of pain due to flare was reported by 11 (68.8%) patients in 28 of 54 (51.9%) treatment cycles (28). Among these patients, 10 (90.9%) had bone metastases, whereas 1 patient probably had non–tumor-related pain due to arthritis in the knees and in the small joints of the fingers. Additional opioid analgesics or dexamethasone was necessitated in 6 patients, one of whom had particularly severe pain. Mild xerostomia, not impairing the quality of life, was observed in 6 (37.5%) patients. Only 1 patient had nausea and short-term vomiting during 1 of 54 (1.9%) treatment applications (Table 4). Xerophthalmia was not reported by any of the patients. No other adverse symptoms were noticed during the entire follow-up period. No grade 2–4 hematotoxicity adverse events occurred. Three patients (18.7%) with disseminated bone metastases had grade 1 hematologic dysfunction already at baseline and after treatment. New grade 1 hematotoxicity was noted in only 1 patient with disseminated bone metastases, whereas absolutely no bone marrow toxicity was observed in 12 patients (75.0%). The median values of hemoglobin, as well as the red blood cell, white blood cell, and platelet counts, did not change significantly during the treatment and follow-up period.
Clinical Reports During 177Lu-PSMA-617 PRLT of Patients with Single Functioning Kidney
Dosimetry of Kidney and Tumors
The single functioning kidney presented a strong physiologic accumulation of 177Lu-PSMA in the posttherapy scans. Renal uptake was characterized by a biexponential curve with rapid decline between the first scan and 3 h after injection, followed by a slower washout. Dosimetry results from 14 patients having undergone 25 cycles of 177Lu PSMA-617 treatment were analyzed. The mean injected activity per cycle was 6.39 ± 1.05 GBq (range, 3.9–8.1 GBq). Calculated radiation-absorbed doses (25) of the kidney per cycle were 5.3 ± 2.1 Gy (0.81 ± 0.32 Gy/GBq). The absolute doses to whole body were 0.35 ± 0.11 Gy (0.058 ± 0.027 Gy/GBq). Mean absorbed doses to tumor lesions were 94.3 ± 23 Gy (13.75 ± 31.59 Gy/GBq).
Renal Toxicity Profile
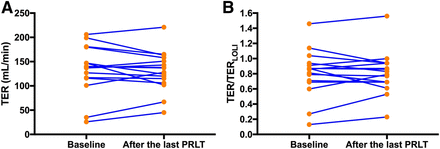
No CTCAE grade 3 or 4 nephrotoxicity was observed during any of the treatment cycles and on a longer follow-up (Figs. 1 and 2). Renal function was already impaired at baseline in 7 (43.8%) patients, according to CTCAE grade 1 in 4 patients (25.0%) and grade 2 in 3 patients (18.8%). Grades 1 and 2 renal functional impairment, respectively, were present in 6 (37.5%) and 1 (6.3%) of the patients after the first PRLT cycle and in 5 (31.3%) and 2 (12.5%) after the second cycle (Fig. 1). TER changed from 135.6 ± 47.3 at baseline to 127.1 ± 41.5 mL/min at follow-up after PRLT, with 10 (62.5%) patients showing a fall in renal function (5 patients > 10%). The TER/TERLoLi ratio changed from 0.81 ± 0.31 to 0.80 ± 0.28. There was no significant change in either TER or TER/TERLoLi ratio after the last cycle of treatment (P = 0.8417) (Fig. 3).
Graphic illustrations of creatinine at baseline and after each therapy according to CTCAE classification: at baseline (A), after first cycle (before second cycle) of treatment (B), after second cycle (before second cycle) of treatment (C), and after third cycle (before fourth cycle) of 177Lu-PSMA PRLT or at restaging (D). No CTCAE grade 3 or 4 impairment was observed.
Quantitative comparison of median serum creatinine at baseline (1) and after each cycle of 177Lu-PSMA-617 PRLT (2–6). Compared with values at baseline, there was no relevant change in means of serum creatinine after treatment.
(A) TER changed from 135.6 ± 47.3 at baseline to 127.1 ± 41.5 mL/min after last treatment, with 10 of 16 (62.5%) patients showing fall in renal function (5/16 > 10%) and 6 of 16 (37.5%) improving. (B) TER/TERLoLi changed from 0.81 ± 0.31 to 0.80 ± 0.28, including 9 of 16 (56.3%) showing fall and 7 of 16 (43.8%) improving. There was no significant change in ratio of TER/TERLoLi before and after last cycle of 177Lu-PSMA-617 treatment (P > 0.05).
In the present study, we further compared the change in TER and TER/TERLoLi ratio before and after treatment. Slightly decreased TERs (2.1%–30.6%) were observed in 10 (62.5%) patients treated with repeated cycles of 177Lu-PSMA-617, whereas 6 (37.5%) patients showed some improvement (2.1%–91.4%) after treatment. Similarly, no significant change in the TER/TERLoLi ratio at baseline and at the end of treatment (P > 0.05) was observed. Slightly decreased TER/TERLoLi ratios (0.9%–29.8%) were noticed in 9 (56.3%) patients treated with repeated cycles of 177Lu-PSMA-617, whereas 7 (43.8%) patients showed some improvement (2.2%–91.4%) after treatment. There was no significant correlation between TER or TER/TERLoLi decrease and the cumulative administered activity (P > 0.05). There was no significant correlation between TER or TER/TERLoLi decrease and the absorbed radiation doses to the kidney (P > 0.05).
Treatment Response
Of the 16 patients, 9 (56.3%) demonstrated a decline in prostate-specific antigen within 2 mo after the first cycle, with 7 (43.8%) showing a prostate-specific antigen decline of at least 50%. On the basis of the EORTC criteria, 7 patients (43.8%) had a partial response and 4 had stable disease (25.0%). The disease control rate after 2 mo (2.1 ± 0.3 mo) after PRLT was 68.8%. According to RECIST 1.1 (CT or MRI), disease control could be reached in 12 patients (disease control rate, 75%), with a partial response in 7 (43.8%) and stable disease in 5 (31.2%), whereas 4 patients (25.0%) had progressive disease.
Outcome and Survival
The median progression-free survival from the commencement of PRLT was 8.1 mo based on both the EORTC criteria and RECIST 1.1 (Fig. 4). By the cutoff date of this study (end of October 2018), 7 (43.8%) of the 16 patients had died, with a median follow-up time of 19.3 mo (range, 5.8–45.3 mo). The median overall survival has yet to be reached (Figs. 4 and 5).
Kaplan–Meier curves for progression-free survival according to EORTC (A), progression-free survival according to RECIST 1.1 (B), and overall survival (C), defined from start of PRLT, for mCRPC patients with single functioning kidney receiving 177Lu-PSMA-617 PRLT.
A 63-y-old man with single functioning kidney and poorly differentiated, castration-resistant, locally advanced prostate cancer with multiple lymph node and bone metastases. Initial tumor classification was pT3bpN1(4/19) M0L1V0Pn1R1. Gleason score was 10 (5 + 5), and initial prostate-specific antigen level was 88 ng/mL. Patient underwent radical prostatectomy, external-beam radiotherapy, androgen deprivation therapy, and chemotherapy between 2012 and 2016 and then progressed in February 2016 with increased prostate-specific antigen of 162 ng/mL. (A, left) Whole-body 68Ga-PSMA PET/CT at baseline. Patient was then treated with 3 cycles of 177Lu-PSMA-617 PRLT with administered radioactivity of 5.6, 5.2, and 6.9 GBq. All treatments were well tolerated. No other adverse symptoms were noticed or reported except pain during entire treatment procedure. (B) SPECT imaging showed high uptake of tumors. Whole-body 68Ga-PSMA PET/CT showed good response (A, before treatment and after 1–3 cycles of 177Lu-PSMA-617 PRLT). Renal function (CTCAE grade 1) did not change before and after treatment, with serum creatinine decrease from 1.37 to 1.15 mg/dL and slight decrease of TER on 99mTc-mercaptoacetyltriglycine renal scintigraphy from 147 to 127 mL/min (C). Prostate-specific antigen level continuously decreased from 162.2 ng/mL in March 2016 to 0.29 ng/mL in December 2017. Patient has achieved 20 mo of progression-free survival until endpoint of study, with good quality of life.
DISCUSSION
Significant PSMA overexpression by mCRPC makes it a promising target for both diagnosis and therapy (theranostics) using radiolabeled small molecules. 177Lu-PSMA PRLT has been demonstrated to be a favorable option especially for end-stage mCRPC, because of the high radiation dose to tumor and sparing of normal organs (29,30). However, PSMA is also expressed in extraprostatic normal tissues such as lacrimal and salivary glands, kidneys, cells of the small intestine, certain neuroendocrine cells in colonic crypts, and celiac ganglia (10,31–33). In kidneys, the apical epithelium of the proximal tubules demonstrate a high PSMA expression that poses a potential risk of nephrotoxicity after PRLT because of the high corresponding accumulation of 177Lu-PSMA (10). There is possibly even a higher risk of toxicity due to the higher radiation burden on a single functioning kidney during PRLT.
Preclinical studies have validated the antagonism of specific PSMA-mediated renal uptake by 2-(phosphonomethyl)pentanedioic acid, a PSMA inhibitor. However, its lack of availability and the possibility of concurrent blockade within the tumor precludes its routine clinical use (34).
We investigated 16 patients with a single functioning kidney treated with at least 2 cycles of 177Lu-PSMA-617 PRLT. Indeed, 3 of the 16 patients had a dysfunctional single kidney due to previous chemotherapy, 4 had hydronephrosis after renal obstruction due to large lymph node masses, 1 had renal metastases, 1 had prevesical ureteral obstruction in the bladder, 2 had an anatomic single kidney after nephrectomy, 1 was possibly due to x-ray contrast–induced nephropathy, 1 had severe hydronephrosis due to postactinic damage of the right ureter, and 3 had severe hydronephrosis due to other reasons. For analysis of adverse renal effects, we investigated the relevant clinical parameters of renal function, that is, serum creatinine, glomerular filtration rate and TER (obtained from 99mTc-mercaptoacetyltriglycine renal scintigraphy), which is also an extremely valuable examination before therapy to rule out any relevant obstructive disease. No CTCAE grade 3 or 4 nephrotoxicity was observed during any cycle of 177Lu-PSMA-617 PRLT or on follow-up. Since 2013, we have not seen any clinically significant nephrotoxicity in long-term follow-up of over 300 patients with both kidneys treated with up to 12 cycles of PRLT. There was no relevant increase in the incidence of acute renal insufficiency in patients with a renal dose higher than 19 Gy in a 2-mo follow-up period (35). The same group found elevated cystatin C in 32 of 55 patients (58%); however, 14 already had elevation of cystatin C before treatment. The renal function significantly correlated with age, hypertension, and prior renal disease (36). No additional nephrotoxicity was observed in patients with a single functioning kidney as compared with patients with both kidneys and with the same level of creatinine at baseline (Supplemental Figure 1; supplemental materials are available at http://jnm.snmjournals.org).
The mean absorbed renal dose was 0.81 ± 0.32 Gy/GBq in these patients with a single functioning kidney, which was similar (0.80 Gy/GBq) to the patient cohort with 2 functioning kidneys receiving PRLT in our center (37). The absorbed renal dose was also consistent with the results by Kabasakal et al. (0.88 ± 0.40 Gy/GBq) (31) and Kratochwil et al. (0.75 Gy/GBq) (38).
Importantly, there was no evidence of any significant hematologic toxicity and, therefore, no possible increased background radiation dose to the red marrow resulting from probable renal dysfunction after PRLT. Physiologic uptake of the PSMA targeting radioligand is also noted in lacrimal glands and salivary glands (39–41). Mild, transient xerostomia was observed in 6 (37.5%) patients (similar to historical controls) without impairing the quality of life. No xerophthalmia occurred in this group.
There was only a mild reduction in TER after PRLT in some patients; however, no significant nephrotoxicity occurred, as also demonstrated by serum creatinine measurements. In the largest group of neuroendocrine tumor patients (n = 807) followed up after PRRT, the incidence of nephrotoxicity was the least in patients receiving 177Lu alone (42). This is relevant in our group of patients receiving 177Lu-PSMA, thereby underlining the fact that 177Lu exhibits no significant nephrotoxicity even in the setting of a single functioning kidney.
This study suffers from a few limitations. The patient number is too small for definite assessment of efficacy. There could be a selection bias in view of the fact that this evaluation was performed on a small group of patients with already advanced disease, for the impact on survival. Despite these shortcomings, we were able to demonstrate for the first time that PRLT is safe, feasible, and perhaps as effective in mCRPC patients with 1 kidney as in those with 2 kidneys. However, further analysis in a larger group of patients with longer follow-up is required.
CONCLUSION
This study demonstrated that in mCRPC patients with a single functioning kidney, 177Lu-PSMA-617 PRLT was feasible, seemed to be effective, and was well tolerated, without any signs of acute or subacute nephrotoxicity, during a mean follow-up of nearly 2 y (and up to 45.3 mo). Further long-term follow-up of this special patient group is warranted.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 177Lu-PSMA radioligand therapy (PRLT) safe and effective in mCRPC patients with a single functioning kidney?
PERTINENT FINDINGS: This cohort study revealed no CTCAE grade 3 or 4 nephrotoxicity and no evidence of any significant hematologic toxicity during or after PRLT in patients with a single functioning kidney. The change in either TER or the ratio of TER to lower-limit TER after 177Lu-PSMA radioligand therapy was not significant, which excludes nephrotoxicity after PRLT.
IMPLICATIONS FOR PATIENT CARE: 177Lu-PSMA radioligand therapy can even be administered in patients with a single functioning kidney due to its safety, especially with regard to the renal function, and long-term efficacy.
Acknowledgments
We thank the referring urologists and oncologists, our physician colleagues, the nursing staff, and the nuclear medicine technologists of Isotope Therapy Ward D3 for patient management.
Footnotes
Published online Mar. 8, 2019.
- © 2019 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 8, 2018.
- Accepted for publication March 4, 2019.