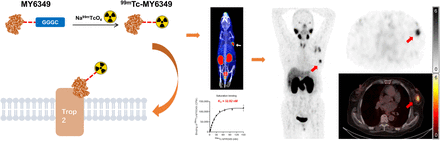
Visual Abstract
Abstract
The trophoblast cell-surface antigen 2 (Trop2) is markedly overexpressed in breast cancers, with a particularly high incidence in triple-negative breast cancer. The therapeutic relevance of Trop2 expression is underscored by the approval of an antibody–drug conjugate for triple-negative breast cancer treatment. However, there is no a predictive technique for accurate whole-body mapping of Trop2 expression in patients. In this study, we developed a novel Trop2-specific molecular probe, [99mTc]Tc-MY6349, and evaluated its safety and feasibility for detecting Trop2 expression in breast cancer using SPECT/CT imaging. Methods: Trop2 expression in different breast cancer cell lines was assessed via immunofluorescence and flow cytometry. The Trop2-specific nanobody MY6349 was site-specifically labeled with 99mTc via a C-terminal GGGC tag, and its binding affinity to the Trop2 receptor was tested in vitro. The in vivo tumor uptake and distribution of [99mTc]Tc-MY6349 were examined through SPECT imaging and biodistribution studies. Furthermore, a pilot clinical study of [99mTc]Tc-MY6349 SPECT/CT was conducted in 8 patients with breast cancer, and the results were compared with [18F]FDG PET/CT. Results: [99mTc]Tc-MY6349 achieved a greater than 95% radiochemical purity after purification. In vitro and in vivo experiments demonstrated the binding specificity of [99mTc]Tc-MY6349 to the Trop2 receptor. In vivo imaging and biodistribution studies revealed a significant correlation between tumor uptake and Trop2 expression levels. In the pilot clinical study, SPECT imaging with [99mTc]Tc-MY6349 successfully detected Trop2-positive tumors 15 min after tracer injection. Delayed imaging showed reduced uptake in normal organs but sustained retention of [99mTc]Tc-MY6349 in tumors. Importantly, [99mTc]Tc-MY6349 SPECT/CT imaging highlighted Trop2 expression heterogeneity and visualized primary and metastatic lesions with a favorable tumor-to-background ratio in breast cancer. Conclusion: [99mTc]Tc-MY6349 was successfully prepared and exhibited a high binding affinity and Trop2 specificity. The pilot clinical study validated the safety and feasibility of [99mTc]Tc-MY6349 SPECT/CT for detecting Trop2 expression in vivo in patients with breast cancer. This imaging modality could complement existing methods, aiding in the guidance of Trop2-targeted therapies and advancing personalized treatment while also promoting the application of SPECT/CT nuclear medicine imaging technology.
Footnotes
Published online Feb. 13, 2025.
- © 2025 by the Society of Nuclear Medicine and Molecular Imaging.
This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
SNMMI members
Login to the site using your SNMMI member credentials
Individuals
Login as an individual user