Abstract
242418
Introduction: The demand for personalized dosimetry-based radiopharmaceutical therapies is growing, but the Single Photon Emission Computed Tomography (SPECT) imaging protocols required are long and pose challenges for routine implementation. Use of Denoising Diffusion Probabilistic Models (DDPM) for the interpolation of projections can be an innovative approach to accelerate SPECT imaging. If scanning time is reduced without compromising image quantification, this will minimize patient discomfort and optimize clinical workflow. This study aims at i) exploring the capabilities of the DDPM model at generating intermediate projections between consecutive acquired ones to cut the duration of a SPECT scan in half, and ii) evaluating the image quantification when image reconstruction including the interpolated projections is performed.
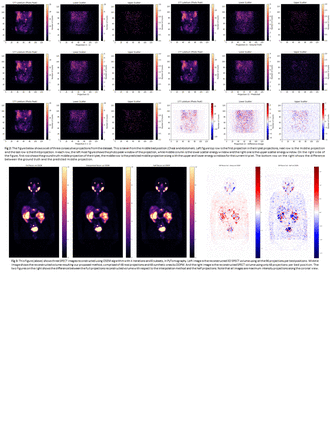
Methods: We used a dataset of 210 177Lu-PSMA-617 SPECT scans from 46 patients with metastatic prostate cancer enrolled in the PR21 Clinical Trial NCT 04663997. Each scan acquired with 3 bed positions, 96 projections per bed. Data were collected with 3 energy windows: Photo peak (PP) (208 keV [187.2 – 228.8]), Lower Scatter (LS) ([166.4 – 187.2] keV), and Upper Scatter (US) ([228.8 – 249.6] keV), each with128x128 pixels resolution. 190 scans of 39 patients were used for train/val, and 20 (7 patients) for test. Each sample is comprised of 3 consecutive projections i-1, i, and i+1, where 1<i<94. The 3 energy windows are stacked to form a three-channel input of size 128x128x3. Frames i-1 and i+1 are provided to a DDPM as prior during training and sampling. Our DDPM involves a U-net architecture with residual blocks and attention with the number of channels = 128 in the first layer. Implementation was done using Python 3.9 and Pytorch 1.9 on a MS Azure VM with a Tesla A-100 16 GB GPU. The model was trained for 500,000 iterations with a linear noise schedule for 1000 steps. During sampling process of DDPM, the model predicts the middle projection for each of the energy windows (PP, LS, US) between projections i-1 and i+1 for all even projections of the test case. The ability of predicting also the LS and US energy windows projections, allows for triple energy window scatter correction. To evaluate the performance of this method, we reconstructed 3 volumes for each test case using: (i) all the measured 96 projections per bed, (ii) the measured even projections per bed (i.e. 48 projections), and (iii) the measured even projections with the DDPM predicted odd projections (i.e. 96 projections per bed). Reconstructions were performed using OSEM algorithm with 4 iterations and 8 subsets by our in-house open source PyTomography package. We evaluated the results using case i as reference with the following metrics: Peak Signal to Noise Ratio, Structural Similarity Index metric, Root Mean Squared Error, and Normalized Mutual Information. Lastly, we compared the activity quantification in the kidneys for the three different reconstructions.
Results: Metrics were very comparable between reconstructed images with method ii and iii (PSNR= 57.6 ± 6.7, SSIM=0.998 ± 0.0020, RSME=0.0017 ± 0.0010, and NMI=0.9994 ± 0.0001), all with respect to reconstruction with method i. Comparison of kidney quantification showed that the median percentage differences were –7.43% [-1.13% to 16.53% to -1.13%] and 0.44% [–11.79% to 6.21%] between images i and iii, and i and ii, respectively.
Conclusions: The similarity metrics suggest that DDPM performs well when generating interpolated projections. The underestimation of activities in the kidneys is most likely due to an overestimation of scatter counts in the US window due to the low statistics in the measured projections. We are applying alternative methods to estimate the US expected to improve quantification accuracy, also extending this work to interpolate a higher fraction of projections, cutting down time further. Overall, it seems feasible to accelerate SPECT acquisitions for routine implementation of dosimetry in the clinic.