Abstract
242499
Introduction: PET data-driven attenuation correction (AC) methods, including deep learning, are a practical option for quantitative brain imaging on standalone brain PET systems and low-dose applications. However, these schemes show activity bias frequently >5%, which is capable of altering study findings. We developed a CT-less transmission-aided AC that combines coincidences from a weak positron source, and the patient, to estimate attenuation with physics alone (Park et al, IEEE Trans. Med. Imaging, 2023). Activity bias was within 5% of CT-AC for phantoms. We aim to assess the performance of this new AC during human brain exams, acquired on whole-body PET/CT.
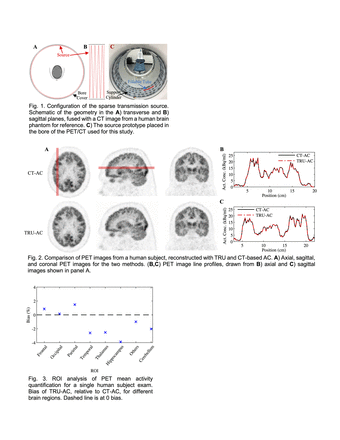
Methods: Our full method, TRansmission-aided μ-map reconstruction (TRU) AC, includes 1) a low-profile and physically fixed transmission source, 2) a modified maximum likelihood reconstruction of attenuation and activity (MLAA) algorithm, and 3) scatter corrections. We implemented a sparse transmission source by wrapping a fillable plastic tube (ID=1.6 mm, total volume=30 ml) around a minimally attenuating hollow cylinder-shaped support (OD=76.2 cm) in a four-turn helix, that spans the PET axial field-of-view. The geometry was chosen to optimize both AC performance and to enable quick filling and placement. The full setup is friction-mounted to the bore cover of the study PET/CT (Siemens Biograph mCT Flow), reducing the bore inner diameter by only 2.8 cm. Our modified MLAA includes a μ-map update that maximizes a penalized log-likelihood of terms representing 1) all coincidences, and 2) hyperparameter weighted counts segmented from the transmission source alone. We estimated scatter from both the transmission source and patient, separately, with single scatter simulations and absolute scaling algorithms. This study was approved by the UT Southwestern IRB. We are recruiting at least N=5 subjects already undergoing 18F-FDG PET/CT imaging as part of their clinical care, and excluding subjects with brain structural pathology (e.g. due to cancer). Patients are injected with 4.8 MBq/kg of 18F-FDG, undergo 60 min of uptake, and a clinical PET/CT, before immediately starting the research scan. The transmission source is filled with 18F-FDG (activity target=15 MBq) and placed in the scanner. The subject is then positioned in the rigid head holder, imaged with CT, and scanned for 10 min with a brain-focused PET exam. For TRU-AC, the CT μ-map for the head holder alone is assumed known (consistent with rigid coils for PET/MR). Otherwise, TRU-AC only uses PET data acquired during the exam intended for AC, without prior image data or μ-map assumptions. PET images are reconstructed with ordered subsets expectation maximization and TRU-AC or CT-AC (reference standard), with all else matched. Brain-structure ROIs are semi-automatically extracted with the MarsBaR extension in SPM12.
Results: We present preliminary findings from a single human subject. A 66 yo male was injected with 444 MBq of 18F-FDG (uptake time=101 min) and imaged with a transmission source activity of 14.5 MBq. Example PET images reconstructed with both CT-AC and TRU-AC show qualitatively strong agreement. This is further supported by line profiles, drawn through both cortical and deep brain structures. For the ROI analysis we found absolute bias in activity concentrations was <3.9% across all brain structures analyzed, relative to CT-AC. Root-mean-square error (RMSE) of activity bias for TRU-AC was 2.8%. Further, the increase in the cerebellar coefficient-of-variation for TRU-AC images was 3.9%, relative to CT-AC. Thus, TRU-AC produced relatively high accuracy and minimal noise amplification.
Conclusions: Our initial results suggest that TRU-AC enables quantitative PET for human neuroimaging. As TRU-AC does not require tracer-specific training data, the scheme may particularly benefit dedicated brain PET exams focused on tracer development, semi-quantitative method validation, new patient cohorts, and/or pathologies that often lack paired CT and PET training data.