Abstract
241348
Introduction: Antibody-based therapies have emerged as a powerful strategy for the management of many diverse cancers, but novel tumor-specific antigens remain challenging to identify and target. Recently, it has been established that inhibitor-modified peptide adducts derived from KRAS G12C are competent for antigen presentation via MHC I and can be targeted by antibody-based therapeutics, offering a means to directly target an intracellular oncoprotein at the cell surface with combination therapies. We have developed antibodies specific to "haptenated" MHC I complexes generated by Sotorasib, an FDA-approved KRAS G12C covalent inhibitor. Here we report a 3.1 Å cryoEM structure of one of these antibodies, P1B7, bound to a Sotorasib-modified, KRAS G12C-derived A*03:01 MHC I complex. We also demonstrate that haptenated MHC I complexes can be leveraged in vivo to recruit radiolabeled P1B7 IgG for tumor imaging with 89Zr (PET) and treatment with 177Lu or 225Ac in combination with Sotorasib treatment, providing superior efficacy than inhibitor monotherapy.
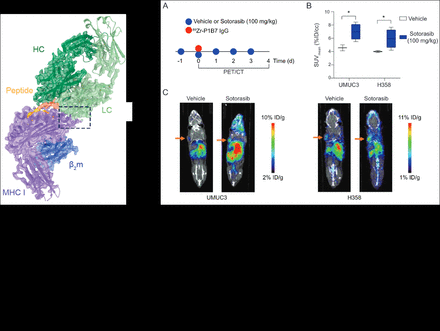
Methods: P1B7 was conjugated to chelators DFO/DOTA/macropa) and subsequently radiolabeled with 89Zr/177Lu/225Ac using previously established protocols. For in vivo imaging studies, mice were treated with 100mpk Sotorasib or saline starting a day before imaging via oral gavage. 110-120 mCi of 89Zr-P1B7 IgG in saline was intravenously injected via tail vein in mice treated with 100 mpk Sotorasib or saline bearing either UMUC3 or H358 tumors (n=4/arm). Mice were imaged on a micro PET/CT scanner at 2h, 4h, 6h, 24h, 48h, 72h, 96h and 120h post injection. At 120 h post injection mice were sacrificed and select organs were harvested. Radioactivity was counted by using an automatic gamma counter, and the radioactivity associated with each organ was expressed as % ID/g. For antitumor assessment studies, mice were split into 4 arms (n=8/arm): saline, Sotorasib (100 mg/kg), 177Lu-P1B7/225Ac IgG (on day 1 and day 7) and Sotorasib (100 mg/kg) + 177Lu-P1B7/225Ac IgG dosed on day 1 and day 7). Mice received either saline or Sotorasib (100 mg/kg) via oral gavage starting on Day 0 and once a day thereafter for two weeks. Mice were monitored for tumor sizes and body weight every other day.
Results: The cryoEM structure provides an atomic understanding of the binding of a Sotorasib-modified KRAS peptide to the A*03:01 MHC I complex and of P1B7’s recognition of this unique composite surface. Radiolabeling with 89Zr/177Lu/225Ac produced the radiochemical complex with > 95% radiochemical yield and purity. Imaging and biodistribution in both UMUC3 and H358 mice revealed higher tumor uptake for mice receiving Sotorasib treatment as compared to those treated with vehicle alone. Antitumor assessment studies with 177Lu-P1B7 revealed that while neither Sotorasib nor 177Lu-P1B7 IgG as single agents significantly impacted tumor growth, the combination of Sotorasib with 177Lu-P1B7 IgG did. Similar but more pronounced effects were observed in 225Ac-P1B7 anti-tumor assessment study. By Day 9, vehicle-treated xenografts had grown to 6x their original size, 225Ac-P1B7 IgG treated xenografts had grown to 4.3x their original size, Sotorasib (30 mg/kg) treated xenografts had grown to 3.1x their original size, but xenografts treated with the 225Ac-P1B7 IgG + Sotorasib combination therapy had only grown to 1.2x their original size. In both studies, all treatments were well tolerated, and no unsafe weight loss was observed in treated mice. Additional control antitumor assessment studies are currently underway, and results will be presented.
Conclusions: In this study, we solved a 3.1 Å CryoEM structure of the P1B7 Sotorasib A*03:01 MHC I complex. We also showed that both 177Lu and 225Ac-labeled P1B7 IgG can significantly reduce tumor growth in KRAS mutant xenograft models when used in combination with Sotorasib providing proof-of-concept in vivo efficacy of an antibody therapeutic targeting Sotorasib-labeled MHC I complexes.