Abstract
242601
Introduction: Resistance to endocrine therapy (ET) is a major cause of mortality in estrogen receptor α (ERα+) positive breast cancer patients. Although disease progression on ET is inevitable in the metastatic setting, benefit is often observed from switching to alternative forms of ET or ET-based approaches. To that end based on preclinical and clinical evidence, the functional status of ER (fER) is thought to be a predictive biomarker of therapeutic outcome. The progesterone receptor (PgR) gene is highly regulated by ER at the mRNA and protein level. Previous clinical studies have demonstrated that changes in 18F-fluorofuranylnorprogsterone (FFNP) uptake after 17β-estradiol (E2) challenge (ΔFFNP-PET) correlated with fER. However, the impact of ESR1 mutations on ΔFFNP is not fully understood. In this work, we evaluate the utility of ∆FFNP as an imaging biomarker for assessing fER in preclinical models with and without mutations in ESR1.
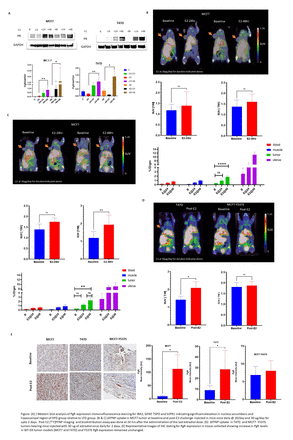
Methods: We pursued both in vitro and in vivo studies using wild type (WT-ER) and Y537S-ER knock-in mutant MCF7 and T47D isogenic cell lines to assess the impact of ESR1 mutation on ΔFFNP. In vitro, cells were cultured in estrogen depleted media and E2 supplemented media for either 24 hrs or 48 hrs. ER and PgR expression levels were determined by Western blotting in cell lysates collected at different time points. In animal studies, 3x106 tumor cells of either MCF7 or T47D were implanted in the second mammary fat pad of ovariectomized immunodeficient mice maintained on E2 water (8 μg/ml). For baseline assessment of PgR, E2 was withdrawn from drinking water 4 days prior to imaging. The ΔFFNP protocol consisted of 20 min static imaging 50 min post injection of FFNP at baseline and 24hr or 48hrs following E2 challenge with subcutaneous injection of either 20 µg/day or 30 µg/day. FFNP-PET was followed by biodistribution assay. FFNP uptake in tumor was quantified by measuring SUVpeak normalized to muscle uptake (T/M ratio). Finally, dynamic PgR expression was assayed by immunohistochemistry (IHC) analysis. Student t-test and two-way ANOVA were performed to compare differences in FFNP-PET uptake between baseline and post-E2 challenge and biodistribution assay, respectively.
Results: In WT-ER MCF7 xenograft, there was a significant (P<0.05) increase (≈ 2 fold) in FFNP uptake at 48hrs following 30 µg/day E2 challenge, but not at 20 µg/day. Further, post-PET biodistribution assay demonstrated statistically significant increase in tumor uptake (P<0.01) from 0.32±0.02 %ID/gram at baseline to 2.02±0.76 % ID/gram at 48 hrs. Since optimal ∆FFNP was observed with 30 µg/day E2 challenge at 48 hrs, we selected this time point for further evaluation of ∆FFNP. In T47D implanted tumors, E2 challenge induced significant (P≤0.05) elevation of FFNP uptake post-E2 (SUVT/M=2.0) compared to the baseline imaging (SUVT/M=1.4). In contrast, ERα mutant MCF-Y537S tumors were impervious to E2 challenge as there was no significant difference between FFNP uptake at baseline (SUVT/M=1.806) and Post-E2 (SUVT/M=1.874). IHC analysis for PgR expression concur with ∆FFNP imaging results with increased staining in WT-ER tumors following E2 challenge but not in Y537S-ER.
Conclusions: These findings confirm that the PgR is regulated by ER and thus PgR expression can serve as an imaging biomarker of fER. As an imaging biomarker of PgR expression, ∆FFNP has provided an effective strategy for assessing fER as a predictive biomarker of clinical response to therapy. Additional studies are warranted to assess the impact of other ERα mutations and the effect of mutant allele fraction in those that fail to show a response in ∆FFNP-PET. The crosstalk of ER and PgR with other pathways and impact of these interactions on ∆FFNP needs to be further evaluated.