Abstract
242584
Introduction: Attenuation of A20 (TNFAIP3), an anti-inflammatory protein which interferes with NF-κB, is significantly influencing inflammation-driven lung cancer growth and metabolism and hence tumor cell behavior. Beside the fact that A20 increases the proliferation of lung adenocarcinoma (LUAD) and energy metabolism, it also affects immune cell infiltration, leading to immune escape. Within the tumor microenvironment (TME), tumor cells and immune cells are active in their metabolism, consuming nutrients and producing metabolites and compete for those. These processes might affect immune cell activity. Therefore, understanding these metabolic changes is key to unraveling the complex interactions within the TME. 2-[18F]fluorodeoxyglucose ([18F]FDG), a glucose-like substance, is essential in PET (Positron Emission Tomography) imaging for cancer staging and monitoring. Although widely used to study various types of tumors, the role of different metabolic activities and distribution in the TME has not been fully explored, mainly due to the limitations of current imaging techniques (e.g.resolution). Our previous work has overcome this by using autoradiography and radioFACS to visualize [18F]FDG uptake in tissues and subcellular fractions1. However, traditional methods of studying tumor metabolism often miss the cellular details of metabolic differences. To overcome these challenges and gain a deeper understanding of tumor metabolism at the cellular level, we aim to include advanced optical techniques. Raman spectroscopy (RS), which provides detailed chemical analysis of biological samples, combined with Optical Coherence Tomography (OCT) allows us to simultaneously study both chemical and structural aspects. This advanced combination, along with PET imaging, not only shows changes in glucose metabolism but also detects alterations in lipids, proteins, and nucleic acids.
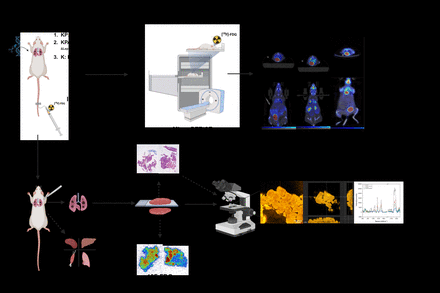
Methods: Autochthonous models were employed to investigate K-ras-driven lung cancer with or without additional floxed A20. Each mouse received [18F]FDG (~10 MBq) via tail vein injection, and either static or dynamic μPET/μCT acquisition was performed. Subsequent, the tumors were harvested and snap-frozen to preserve their integrity. A part of the lung tissue was used for autoradiography. The residual lung tumor tissue underwent a comprehensive analysis: First, Hematoxylin and Eosin (H&E) staining was applied for guiding further imaging analysis and define tumor regions. The H&E-stained sections were then subjected to multiple imaging modalities, including Raman Spectroscopy (RS) and Optical Coherence Tomography (OCT) (See Figure 1 for the workflow).
Results: The average SUVmax of the lung in KPA mice (2.34) was significantly lower than in KP mice (3.36, P=0.03). Illustrative PET images of KP and KPA models are displayed (Fig. 2a,d) Autoradiographic images (Fig. 2b,e) highlight a heterogeneous radioactivity distribution in the lungs and tumor areas, indicating variable metabolic activity of tumor cells and uneven [18F]FDG distribution. Raman spectral analysis of KP and KPA tumors shows differences in peak intensities and positions, emphasizing protein (1500-1700 cm−1) and lipid (2700-3000 cm−1) presence in the tumors, indicating shifted protein and lipid metabolism between the groups.
Conclusions: This study demonstrates the power of multi-modal molecular imaging to reveal intricate metabolic changes resulting from A20 downregulation in lung adenocarcinomas, offering crucial insights for targeted therapies and advancing cancer metabolism research. Hence, this work represents a major step forward in understanding the complex metabolic diversity of tumors.