Visual Abstract
Abstract
6-(fluoro-18F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([18F]MK6240) has high affinity and selectivity for hyperphosphorylated tau and readily crosses the blood–brain barrier. This study investigated whether the early phase of [18F]MK6240 can be used to provide a surrogate index of cerebral perfusion. Methods: Forty-nine subjects who were cognitively normal (CN), had mild cognitive impairment (MCI), or had Alzheimer’s disease (AD) underwent paired dynamic [18F]MK6240 and [11C]Pittsburgh compound B (PiB) PET, as well as structural MRI to obtain anatomic information. Arterial blood samples were collected in a subset of 24 subjects for [18F]MK6240 scans to derive metabolite-corrected arterial input functions. Regional time–activity curves were extracted using atlases available in the Montreal Neurologic Institute template space and using FreeSurfer. The early phase of brain time–activity curves was analyzed using a 1-tissue-compartment model to obtain a robust estimate of the rate of transfer from plasma to brain tissue, K1 (mL⋅cm−3⋅min−1), and the simplified reference tissue model 2 was investigated for noninvasive estimation of the relative delivery rate, R1 (unitless). A head-to-head comparison with R1 derived from [11C]PiB scans was performed. Grouped differences in R1 were evaluated among CN, MCI, and AD subjects. Results: Regional K1 values suggested a relatively high extraction fraction. R1 estimated noninvasively from simplified reference tissue model 2 agreed well with R1 calculated indirectly from the blood-based compartment modeling (r = 0.99; mean difference, 0.024 ± 0.027), suggesting that robust estimates were obtained. R1 measurements obtained with [18F]MK6240 correlated strongly and overall agreed well with those obtained from [11C]PiB (r = 0.93; mean difference, −0.001 ± 0.068). Statistically significant differences were observed in regional R1 measurements among CN, MCI, and AD subjects, notably in the temporal and parietal cortices. Conclusion: Our results provide evidence that the early phase of [18F]MK6240 images may be used to derive a useful index of cerebral perfusion. The early and late phases of a [18F]MK6240 dynamic acquisition may thus offer complementary information about the pathophysiologic mechanisms of the disease.
Developed as a second-generation tau tracer, 6-(fluoro-18F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([18F]MK6240) possesses high affinity and selectivity toward hyperphosphorylated tau (1–4), one of the hallmark neuropathologies in Alzheimer’s disease (AD). Initial pharmacokinetic studies with arterial blood sampling also indicated a relatively fast brain penetration, as evidenced by moderately high rates of transfer from plasma to brain tissue (K1) (2,3,5)—the K1 from plasma to brain tissue that is related to cerebral blood flow (CBF) as the product of CBF and extraction (E), such that K1 = CBF × E = CBF × [1 − e−PS/CBF], where PS is the vascular permeability–surface area product. The dynamic range of K1 estimates across brain regions previously reported (2,3,5) suggests that the early phase of dynamic [18F]MK6240 PET measurements might be used to provide valuable information on cerebral perfusion.
A deficit in cerebral perfusion has been well documented in AD, with a reduction in CBF reported for several cortical regions, such as the frontal, parietal, and temporal cortices (6–10). Decreased CBF has also been reported in other tauopathies, as well as in healthy elderly subjects when compared with young adults (11–14). Although it is not clear whether hypoperfusion plays a primary or secondary role in AD and related dementia, hypoperfusion is an important component to understanding the pathogenesis of these neurodegenerative diseases and may also be an important prognostic biomarker or treatment target.
Several imaging modalities have been used for in vivo measurements of CBF, including MR-based methods such as arterial spin labeling, but the gold standard technique remains [15O]H2O PET analyzed by tracer kinetic modeling. [15O]H2O possesses attractive properties for measuring blood flow, with a high extraction fraction for a wide range of flow values (15,16). However, its short half-life (∼2 min) requires an on-site cyclotron, and performing an additional scan to obtain CBF measurements may be cumbersome, particularly in a clinical setting, potentially leading to logistic complications. Alternatively, measuring CBF using arterial spin labeling techniques would require either a simultaneous PET/MRI scanner or a separate MRI scan including additional sequences. These reasons have led several investigators to study whether the early phase of the PET acquisition of amyloid or other tau tracers (17–22) can be used to provide surrogate measurements of cerebral perfusion or relative delivery rate (R1), such that R1 = K1 (target)/K1 (reference region). Since CBF is generally tightly coupled to brain metabolism, several investigators have also performed head-to-head comparisons with [18F]FDG, proposing that this early phase might be used to provide a surrogate biomarker of neuronal injury (23–29), thus providing information on “N” (neurodegeneration) in addition to “A” (amyloid) or “T” (tau) in the A/T/N classification scheme (30,31).
In this article, we seek to present the first supporting evidence that the early phase of [18F]MK6240 can be used to provide a surrogate index of cerebral perfusion. Our hypothesis is that the early phase of [18F]MK6240 images can be used to provide quantitative information on cerebral perfusion in addition to a measure of tau load—typically obtained by a full kinetic analysis or derived from a late acquisition after tracer injection—thus augmenting the utility of this tracer. Since other investigators have shown that dynamic [11C]Pittsburgh compound B (PiB) scanning may be used to measure changes in perfusion and that R1 obtained with [11C]PiB correlates well with R1 obtained from [15O]H2O scans (17,18), and in the absence of [15O]H2O measurements, we also performed a direct comparison between R1 obtained with [18F]MK6240 and R1 obtained from corresponding [11C]PiB scans.
MATERIALS AND METHODS
A full version of the Materials and Methods section is provided in the supplemental materials (available at http://jnm.snmjournals.org).
Participants
In total, 49 subjects, consisting of 25 who were cognitively normal (CN), 17 who had mild cognitive impairment (MCI), and 7 who had AD, were included in this work. All subjects were identified by physicians at Massachusetts General Hospital and underwent at least one comprehensive medical and neurologic evaluation using tests that included the Mini-Mental State Examination. Clinical status (MCI, AD, or CN) was determined clinically by established criteria (32). All subjects signed an informed-consent form, and the study was approved by the institutional review board at Massachusetts General Hospital.
Data Acquisition
Each subject underwent a dynamic [18F]MK6240 PET scan for up to 135 min, a dynamic [11C]PiB PET scan for 60 min, and MRI for anatomic reference. A subset of 24 [18F]MK6240 scans included arterial blood sampling (14 CNs, 8 MCIs, and 2 ADs). [18F]MK6240 and [11C]PiB were synthesized as previously described (33,34). Acquisition of [18F]MK6240, [11C]PiB, and MRI data was also previously described (2,35).
Image Processing
Dynamic PET images were motion-corrected using validated tools (36–38), and regional time–activity curves were extracted using the Montreal Neurologic Institute (MNI) template space (2,39) and FreeSurfer (40,41). Time–activity curves extracted using the MNI atlases were used primarily for comparisons between outcome measures while grouping all regions of interest and subjects on the same graphs. Time–activity curves resulting from the FreeSurfer parcellation were used primarily for detailed regional comparisons. PiB scores were calculated using the method of Johnson et al. (42)
Quantitative Analysis
Blood-Based Compartmental Analysis
The early phase of [18F]MK6240 regional time–activity curves was analyzed using a simple 1-tissue-compartment model with 2 rate constants k (K1 and k2) and including the vascular fraction (v) as an additional model parameter (1T2kv). The time stability of K1 and R1 estimates was evaluated by comparing values obtained using 5 and 10 min of data. In this work, we followed the consensus nomenclature for imaging of reversibly binding radioligands described by Innis et al. (43)
Simulation Study
Simulations were performed with different levels of specific binding to assess the effect of neglecting the binding component on K1 and R1 estimates obtained from the 1T2kv model. Regional 120-min time–activity curves were simulated for the gray cerebellum (reference region) and target regions using a 2-tissue-compartment model applying arterial measurements and kinetic parameters derived from human studies. Simulated time–activity curves were truncated, and the first 5 or 10 min of data were analyzed with the 1T2kv model. K1 and R1 estimates were then compared with simulated ground truth values.
Reference Region–Based Analysis
Noninvasive measurements of R1 were investigated using the simplified reference tissue model 2 (SRTM2) (44) and compared with those obtained indirectly from the blood-based compartmental analysis. The effect of using different strategies for fixing the reference region clearance constant,  , on the estimation of R1 was investigated.
, on the estimation of R1 was investigated.
Head-to-Head Comparison with [11C]PiB
A direct comparison between R1 obtained with [18F]MK6240 and R1 obtained from corresponding [11C]PiB scans was performed with SRTM2 using the first 5 min of data.
Regional Analysis of R1 Between Groups
Differences in R1 were evaluated among CN, MCI, and AD subjects. The same analysis was also repeated while grouping MCI and AD subjects.
Statistical Analysis
The Pearson correlation coefficient r was used to assess the strength of the linear correlations between outcome measures. All data were expressed as mean ± SD unless otherwise specified. A P value of 0.05 or less was considered statistically significant. Bonferroni adjustment was applied for multiple comparisons on the same datasets when appropriate. More details on statistical analysis are provided in the supplemental materials.
RESULTS
Participants
Subject demographics are summarized in Table 1. All CN subjects (n = 25) had a Mini-Mental State Examination result of at least 25. Other participants met the National Institute of Aging research criteria for MCI or for AD dementia. Twenty-five subjects (8 CNs, 10 MCIs, and all 7 ADs) were amyloid-β–positive as defined by a distribution volume ratio of more than 1.2 calculated in a large cortical ROI that included the frontal, lateral temporal, and retrosplenial cortices (45). The CN and MCI/AD groups were well matched in age (68.7 ± 12.5 y vs. 69.8 ± 11.0 y, P = 0.7513; unpaired 2-sample t test). The time between [18F]MK6240 and [11C]PiB scans was variable between subjects: 29 subjects had their [11C]PiB and [18F]MK6240 scans performed within 2 wk of each other (18 of which had them on the same day), and for the remaining subjects the 2 scans were separated by up to several months. Thirty-five subjects had their [11C]PiB scan performed before their [18F]MK6240 scan, and the remaining subjects had their [18F]MK6240 scan performed first.
Participant Demographics and Clinical Characteristics
Blood-Based Compartmental Analysis
The K1 estimates obtained from the 1T2kv model were stable while using 5 or 10 min of data, indicating the robustness of these measurements and thus demonstrating that the estimation of K1 is sufficiently decoupled from the estimation of the parameter k2. The total-least-square regression and Bland–Altman plots demonstrated a good correlation and agreement, respectively (y = 1.02x − 0.01, r = 0.99, P < 0.0001; mean difference, 0.000 ± 0.009 mL⋅cm−3⋅min−1; P = 0.9521) and also highlight the relatively broad range of K1 values measured across subjects and brain regions (Supplemental Fig. 1). Similar levels of correlation and agreement were observed when comparing corresponding R1 estimates (y = 1.01x − 0.01, r = 0.99, P < 0.0001; mean difference, −0.004 ± 0.019; P = 0.8026). On average, cerebellar K1 values were lower in the MCI/AD group than in the CN group (0.326 ± 0.066 mL⋅cm−3⋅min−1 vs. 0.391 ± 0.094 mL⋅cm−3⋅min−1); however, these differences were not statistically significant as assessed by a 2-tailed unpaired t test (P = 0.0767).
Simulations at Different Levels of Specific Binding
Supplemental Table 1 shows the results of the simulation study performed for 3 different R1 values (0.4, 0.85, and 1.3) at increasing levels of specific binding in the target region as indicated by the simulated range of distribution volume ratios. When only the first 5 min of simulated time–activity curves were fitted, the bias in the estimated K1 and corresponding R1 in the target region was well below 2% for all levels of specific binding. When the first 10 min of time–activity curves were fitted, the bias in K1 and R1 estimates in the target region increased with the level of specific binding and was higher for larger simulated values of R1 (−7.06% and −6.12%, respectively, at R1 = 1.3). The measured bias on estimated K1 in the reference region was 0.04% and 1% when using 5 min and 10 min of data, respectively.
Reference Region Model-Based Analysis
Direct estimates of R1 obtained from SRTM2 showed a strong correlation and good agreement (y = 0.95x + 0.07, r = 0.99, P < 0.0001; mean difference, 0.024 ± 0.027; P = 0.1261) with those obtained indirectly from the blood-based compartmental analysis using the first 5 min of PET data for each method (Fig. 1). The mean subject-specific median  value across subjects was 0.084 ± 0.049 min−1 (range, 0.022–0.270 min−1). Fixing
value across subjects was 0.084 ± 0.049 min−1 (range, 0.022–0.270 min−1). Fixing  to a population-based value determined from SRTM (
to a population-based value determined from SRTM ( ) had a negligible effect on R1 estimates in comparison to using subject-specific median
) had a negligible effect on R1 estimates in comparison to using subject-specific median  values (mean difference across all subjects and brain regions, −0.06% ± 0.40%). Likewise, fixing
values (mean difference across all subjects and brain regions, −0.06% ± 0.40%). Likewise, fixing  to
to  blood-based (the average cerebellar blood-based
blood-based (the average cerebellar blood-based  estimates, with
estimates, with  blood-based = 0.062 ± 0.014 min−1 and range = 0.040–0.088 min−1) led to similar R1 estimates in comparison to using subject-specific median
blood-based = 0.062 ± 0.014 min−1 and range = 0.040–0.088 min−1) led to similar R1 estimates in comparison to using subject-specific median 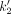 values (mean difference across all subjects and brain regions, −0.08% ± 0.33%). Therefore, for all SRTM2 analyses performed with [18F]MK6240 in this work, we used
values (mean difference across all subjects and brain regions, −0.08% ± 0.33%). Therefore, for all SRTM2 analyses performed with [18F]MK6240 in this work, we used  . The SRTM2 R1 estimates were also stable when using 10 min of data compared with using only 5 min (y = 1.00x − 0.02, r = 0.99, P < 0.0001; mean difference, −0.013 ± 0.019; P = 0.2522).
. The SRTM2 R1 estimates were also stable when using 10 min of data compared with using only 5 min (y = 1.00x − 0.02, r = 0.99, P < 0.0001; mean difference, −0.013 ± 0.019; P = 0.2522).
Comparison of direct R1 estimates obtained from SRTM2 and indirect blood-based R1 estimates obtained from 1T2kv model with [18F]MK6240. (A) Correlation plot. Red line represents total-least-square regression, and black line is line of identity. (B) Corresponding Bland–Altman plot showing agreement in R1 estimates between 2 methods. Solid red line represents mean difference, dashed red lines show mean difference ± 1.96 SD, and black line is zero line for reference. Blue dots represent CN subjects, and orange dots represents MCI/AD subjects. Data points are for all subjects, and brain time–activity curves were surveyed using atlases in MNI template space.
Head-to-Head Comparison Between R1 Estimates Obtained from [18F]MK6240 and [11C]PiB
Figure 2 shows a direct comparison between R1 estimates obtained from [18F]MK6240 and [11C]PiB scans. When all 49 paired scans were included in the analysis, the overall correlation and agreement were good despite some variability (Figs. 2A and 2B: y = 0.97x + 0.03, r = 0.93, P < 0.0001; mean difference, −0.001 ± 0.068; P = 0.9242). When the analysis was restricted to pairs of [18F]MK6240 and [11C]PiB scans acquired within 2 wk, the correlation and agreement were slightly better (Figs. 2C and 2D: y = 0.99x + 0.00, r = 0.95, P < 0.0001; mean difference, −0.003 ± 0.059; P = 0.8623), possibly reflecting daily variations in CBF. Restricting the analysis to pairs of scans acquired the same day further reduced the variability (Figs. 2E and 2F: y = 0.99x + 0.00, r = 0.97, P < 0.0001; mean difference, −0.010 ± 0.043; P = 0.6273).
Comparison of SRTM2 R1 estimates obtained from [18F]MK6240 and [11C]PiB paired scans. (A and B) Correlation and Bland–Altman plots of R1 estimates for all paired [18F]MK6240 and [11C]PiB scans (49 pairs). (C and D) Correlation and Bland–Altman plots of R1 estimates for paired scans acquired within 15 d (29 pairs). (E and F) Correlation and Bland–Altman plots of R1 estimates for paired scans acquired on same day (18 pairs). In correlation plots (A, C, and E), solid red lines represent total-least-square regressions and black lines are lines of identity. In Bland–Altman plots (B, D, and F), solid red lines represent mean difference, dashed red lines show mean difference ± 1.96 SD, and black lines are zero lines for references. Blue and orange dots represent brain regions corresponding to CN subjects and MCI/AD subjects, respectively. Data points are for all subjects, and brain time–activity curves were surveyed using atlases in MNI template space.
Regional differences in R1 estimates obtained from [18F]MK6240 and [11C]PiB scans for the FreeSurfer analysis are presented in Supplemental Table 2. After Bonferroni adjustment for multiple comparisons, none of the observed differences were statistically significant when grouping all subjects together or when considering CN subjects only. In the MCI/AD group, only differences in the inferior temporal cortex survived the Bonferroni adjustment. Overall, these results demonstrate that similar R1 estimates were obtained from [18F]MK6240 and [11C]PiB scans.
Regional Analysis of R1 Among Groups
Figure 3 shows the average regional R1 values measured in each group for regions of interest derived from the FreeSurfer parcellation. All P values for regional comparisons can be found in Supplemental Table 3. Figure 3A shows bar plots with CN, MCI, and AD groups presented separately. When MCI and CN subjects or MCI and AD subjects were compared, none of the measured differences that were below a P value of 0.05 survived Bonferroni adjustment. When AD and CN subjects were compared, only the inferior-temporal cortex, middle-temporal cortex, and inferior-parietal cortex were still statistically significant after Bonferroni adjustment. Finally, when MCI and AD subjects were grouped and compared with CN subjects, only differences in the middle-temporal cortex survived Bonferroni adjustment (Fig. 3B).
Bar plots showing differences in regional R1 measurements among CN, MCI, and AD groups for analysis performed using FreeSurfer parcellation. (A) Plots with CN, MCI, and AD groups presented separately. (B) Plots presented while grouping MCI and AD groups. Asterisks indicate differences that were statistically significant between groups as assessed from unpaired 2-tailed t test assuming unequal variance and after Bonferroni adjustment (P < 0.0013). Asterisks in A resulted from comparison between CN and AD groups. Asterisk in B resulted from comparison between CN and MCI/AD groups. Error bars represent SD measured within each group.
Parametric Imaging
Figure 4 shows parametric images of R1 computed with SRTM2 using 10 min of dynamic data, along with the structural MRI for anatomic reference, the [11C]PiB distribution volume ratio images showing amyloid burden, and the late [18F]MK6240 SUV ratio for images from 90 to 120 min showing tau pathology. The figure shows a side-by-side comparison between a CN subject and a subject with AD of similar age. Although the CN subject showed relatively homogeneous perfusion in the cortical and subcortical areas, the AD subject showed marked hypoperfusion in the temporal and parietal cortices, which were also areas showing tau pathology. Group-average images of [18F]MK6240 R1 are shown in Figure 5. Visually, a reduction in R1 was apparent on the AD-average images as compared with CN and MCI subjects.
Parametric maps and corresponding MR structural images in CN subject and AD subject. First row shows magnetization-prepared rapid gradient echo as anatomic reference. Second row shows parametric [11C]PiB DVR images as measure of amyloid burden. Third row shows late [18F]MK6240 SUV ratio for images from 90 to 120 min as measure of tau load. Fourth row shows parametric images of R1 computed from [18F]MK6240 dynamic data. Arrows indicate areas of reduced cerebral perfusion and corresponding to high tau load. MPRAGE = magnetization-prepared rapid gradient echo; DVR = distribution volume ratio; SUVR = SUV ratio; SUVR90–120 = SUV ratio for images from 90 to 120 min.
Group-average images of [18F]MK6240 R1 in CN, MCI, and AD subjects. Images are presented in MNI template space. Corresponding slices of MNI template are shown for anatomic reference.
DISCUSSION
In this work, we investigated the use of early-phase [18F]MK6240 dynamic images to derive an index of relative perfusion by tracer kinetic modeling. We chose to fit only the first few minutes of PET measurements since this early phase after tracer injection is the most sensitive part of the scan to changes in perfusion. Mathematically, this can be demonstrated by plotting the sensitivity curves of the model parameters (Supplemental Fig. 2). Our simulation results demonstrated that fitting the first 5 min of PET data with a simple 1-tissue-compartment model provided K1 and R1 estimates within 2% of ground truth, suggesting that neglecting specific binding does not introduce noticeable bias or variability. Using 10 min of data, however, resulted in a slightly higher bias that increased with increasing level of specific binding (i.e., increasing distribution volume ratios) and with simulated R1 values. Nevertheless, we generated R1 parametric images using 10 min of data because the quality of the parametric maps was higher than when using only 5 min after tracer injection (Supplemental Fig. 3), thus accepting a tradeoff between bias and image quality. Overall, our findings from simulations and experimental data suggest that [18F]MK6240 can provide robust surrogate measurements of relative perfusion. We found good agreement between direct estimates of R1 obtained by SRTM2 and indirect R1 estimates from blood-based compartment modeling, as well as good agreement between [18F]MK6240 and [11C]PiB R1 estimates.
Quantitative measurements of perfusion derived from the early phase of [11C]PiB have previously been compared with the gold standard, [15O]H2O, by several investigators who reported relatively high values of the K1 rate constant and subsequently proposed that [11C]PiB might also be used to deduce changes in blood flow (17,18,46). Gjedde et al. previously presented evidence that the early phase of [11C]PiB is not completely flow-limited, with significant but not unlimited permeability, and reported an average extraction fraction of 0.53 by comparing K1 with CBF as measured by [15O]H2O in different brain regions of AD and CN subjects (17). Subsequent work by Chen et al. reported that R1 derived from SRTM2 correlated strongly with regional relative [15O]H2O CBF measurements (18), and more recently, Heeman et al. demonstrated excellent test–retest reproducibility of R1 measurement with [11C]PiB, suggesting suitability for cross-sectional and longitudinal studies (47). Of note, the regional K1 estimates and dynamic range of values measured in CN subjects in the present work with [18F]MK6240 were about 20%–25% higher than those previously reported for [11C]PiB in healthy volunteers of comparable age (18), suggesting that [18F]MK6240 may possess a higher extraction fraction and therefore may be more sensitive to changes in CBF—although data were acquired on different scanners, using different reconstruction algorithms and different brain atlases for time–activity curve extraction, thus precluding a definitive conclusion. When we normalized by the gray cerebellum, R1 values derived by [18F]MK6240 and [11C]PiB scans were similar, suggesting comparability of R1 measurements between these 2 tracers for cross-sectional or longitudinal studies. In light of these findings and previous work with [11C]PiB, future studies including head-to-head comparisons between [18F]MK6240 and [15O]H2O with arterial blood sampling would be necessary to determine the extraction fraction of [18F]MK6240 and to evaluate its actual sensitivity to small changes in CBF by direct comparison of regional K1 values between the 2 tracers and across CN, MCI, and AD subjects.
In the present work, we performed a group comparison of R1 among CN, MCI, and AD subjects using regions of interest derived from the parcellation provided by FreeSurfer. The differences between groups were generally consistent with perfusion deficits generally reported by previous work using reference techniques (6), although some discrepancies exist in the literature possibly due to differences in methodology and cohorts across studies.
Although a full 120-min dynamic PET acquisition is ideal for providing fully quantitative measurements of tau pathology and indices of cerebral perfusion by tracer kinetic modeling, such a long dynamic scanning protocol is impractical in a clinical setting and typically results in patient discomfort and reduced scanning efficiency compared with shorter static scans. To address this limitation, and similarly to what was previously proposed for amyloid imaging (48), Kolinger et al. recently proposed a dual-time-window acquisition protocol for accurate quantitative measurements of longitudinal changes in tau load for [18F]MK6240 studies (49). Such a protocol would be particularly suited to allow for both quantification of tau pathology—either using kinetic analysis to derive a quantitative distribution volume ratio or using a semiquantitative late SUV ratio—and derivation of an index of cerebral perfusion such as proposed in the current study.
One of the limitations of the present work is the relatively small sample size for the AD group. For this reason, in our group analyses we also presented results grouping MCI and AD subjects and comparing them with CN subjects. Our data were also acquired on 2 different systems. Although both scanners possess a similar intrinsic spatial resolution (2) and were calibrated and validated for absolute quantification, no further efforts were made to specifically harmonize the 2 scanners to each other. However, for each participant, paired [18F]MK6240 and [11C]PiB scans were performed on the same scanner and reconstructed with the same reconstruction algorithm; therefore, our results comparing the R1 for [18F]MK6240 and [11C]PiB should be largely unaffected by the type of scanner. Another limitation relates to the use of a reference tissue model for deriving R1. Although reference region techniques present the advantage of being noninvasive, that is, not requiring arterial cannulation and blood sampling, they provide a measure of only relative perfusion and cannot capture global changes in perfusion. In the present work, we used the gray cerebellum as a reference region, and although we did not find statistically significant differences in cerebellar K1 at the group level among CN, MCI, and AD subjects, there were nonetheless some differences among subjects. Consequently, after normalization by cerebellar K1, some group differences between the CN and MCI/AD groups that were due to global changes in perfusion (as observed in Supplemental Figs. 1A and 1B) were not apparent in R1 estimates (Supplemental Figs. 1C and 1D). This is, however, consistent with the observation from previous studies that AD is associated with both global and regional cerebral hypoperfusion (6).
CONCLUSION
Our results support use of the early phase of [18F]MK6240 images to derive a quantitative index of cerebral perfusion. The early and late phases of [18F]MK6240 dynamic acquisitions may thus offer complementary information on cerebral perfusion and tau load, respectively. Further work including a direct comparison with the gold standard, [15O]H2O, will be needed to determine the extraction fraction of [18F]MK6240 and sensitivity to small changes in CBF at different flow values.
DISCLOSURE
This study was funded by the Massachusetts General Hospital Thrall Innovation award (Nicolas Guehl); grant R21AG070714 (Nicolas Guehl); grants S10OD018035, R01AG076153, and P41EB022544 (Georges El Fakhri); and grant R01AG046396 (Keith Johnson). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can the early phase of [18F]MK6240 dynamic imaging be used to derive a surrogate index of relative cerebral perfusion?
PERTINENT FINDINGS: Robust estimates of R1 were obtained. The head-to-head comparison of [18F]MK6240 and [11C]PiB showed similar measurements of R1, suggesting that they provide similar information on relative cerebral perfusion. Direct comparison with [15O]H2O is needed to characterize the extraction fraction of [18F]MK6240 and its sensitivity to small changes in CBF.
IMPLICATIONS FOR PATIENT CARE: The early and late phases of [18F]MK6240 dynamic scanning may offer complementary pathophysiologic information in AD, thus providing indices of relative cerebral perfusion and tau load, respectively.
ACKNOWLEDGMENTS
We thank Julia Scotton and Nicole DaSilva for preparing the subjects, scanning, and monitoring, and we thank Steven Weise and Marina MacDonald-Soccorso for helping with data management.
Footnotes
Published online Mar. 30, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 21, 2022.
- Revision received January 26, 2023.















