Visual Abstract
Abstract
Dosimetry after 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) enables estimation of radiation doses absorbed by normal organs and target lesions. This process is time-consuming and requires multiple posttreatment studies on several subsequent days. In a previous study, we described a newly developed multiple-linear-regression model to predict absorbed doses (ADs) from a single-time-point (STP) posttreatment study acquired 168 h after the first infusion and 24 h after the following ones, with similar results to the standard multiple-time-point (MTP) protocol. The present study aimed to validate this model in a large patient cohort and to assess whether STP dosimetry affects patient management decisions compared with our MTP protocol. Methods: Quantitative 177Lu-DOTATATE SPECT/CT post-PRRT data from 159 consecutive patients (172 therapies, 477 therapy cycles) were retrospectively analyzed. ADs obtained from an STP model were compared with those obtained using an MTP model. We evaluated the impact of the STP model on the decision on whether PRRT should be stopped because of an expected kidney AD exceeding the safety threshold. We hypothesized that patient management based on the STP model does not differ from that based on the MTP model in at least 90% of the cases. Results: There was no difference in management decisions between the MTP and STP models in 170 of 172 therapies (98.8%). A Fisher χ2 test for combined probabilities produced a composite P value of 0.0003. Mean cumulative AD relative differences between the STP and MTP models were 0.8% ± 8.0%, −7.7% ± 4.8%, 0.0% ± 11.4%, −2.8% ± 6.3%, and −2.1% ± 18.4% for kidneys, bone marrow, liver, spleen, and tumors, respectively (Pearson r = 0.99 for all), for patients who underwent 4 therapy cycles. Similar results were obtained with fewer therapy cycles. Conclusion: Estimated radiation ADs and patient management decisions were similar with the STP and MTP models. The STP model can simplify the dosimetry process while also reducing scanner and staff time and improving patient comfort.
Peptide receptor radionuclide therapy (PRRT) with the radionuclide 177Lu-DOTATATE is effective in the management of neuroendocrine neoplasms (1–3). Currently, PRRT is administered following an empiric protocol of 4 fixed doses of 7.4 GBq (200 mCi) of 177Lu-DOTATATE, as approved by the Food and Drug Administration (4). However, because the absorbed dose (AD) to critical organs is highly variable, the therapy dose or number of therapy cycles might be increased, thereby increasing the AD to tumor sites without exceeding the critical healthy-organ safety thresholds (5,6). Several studies have assessed personalized PRRT based on the patient-specific AD (5–8)—that is, adjusting the number of therapy cycles—with low toxicity and promising efficacy (6,8).
Dosimetry calculation after PRRT is essential, but the process requires multiple posttreatment SPECT acquisitions corrected for photon attenuation (using CT attenuation maps) on several subsequent days, followed by complex image processing and calculation of ADs. The European Association of Nuclear Medicine/MIRD guidelines for quantitative 177Lu imaging (9) require 3 quantitative SPECT/CT studies at a time 1 (t1) of 24 h, a time 2 of 96 h, and a time 3 (t3) of 168 h after the first treatment cycle and a single SPECT/CT examination at t1 after the following cycles. In a recent study (10), we trained a multiple-linear-regression (MLR) model on a set of 40 consecutive patients for prediction of radiation ADs using a single posttreatment SPECT/CT study performed at a t3 of 168 h after the first therapy cycle and at a t1 of 24 h after the subsequent cycles, with small mean relative differences from our standard multiple-time-point (MTP) protocol for kidneys, bone marrow, liver, spleen, and tumor sites. The aim of the present study was to confirm the accuracy of single-time-point (STP) dosimetry in a large patient cohort receiving PRRT and to define whether it guides management decisions similarly to the results obtained with MTP measurements.
MATERIALS AND METHODS
Patient Population
Between November 2011 and March 2022, 297 consecutive patients with neuroendocrine neoplasms received 1,041 PRRT treatment cycles with 177Lu-DOTATATE at our institution.
The study inclusion criteria were, first, patients who started and completed their series of treatments during the defined period; second, patients who underwent MTP dosimetry following our standard protocol, including 3 quantitative SPECT/CT studies at a t1 of 24 h, a t2 of 98 h, and a t3 of 168 h or 2 SPECT/CT studies at t1 and t3 after the first therapy cycle and a single SPECT/CT study at t1 after the subsequent cycles; and third, patients who were not included in the training dataset used to generate the STP MLR model (10).
A total of 281 patients had PRRT during this period. Three patients were excluded because of missing data in the hospital archiving system. Of 278 remaining patients, 178 underwent MTP dosimetry with SPECT/CT in the appropriate acquisition times described above. Nineteen patients, included in the training dataset of the previous study, were excluded from the present study. The remaining 159 patients (95 men; average age, 60 y; range, 12–88 y) with 477 therapy cycles (5 cycles in 2 patients, 4 cycles in 57, 3 cycles in 45, 2 cycles in 36, and 1 cycle in 32) and a total of 172 PRRTs (13 salvage therapies) were included in this study. In 107 patients who did not complete 4 cycles of PRRT, 27 (25%) stopped receiving the therapy because the expected kidney dose after the following cycle exceeded 25 Gy, 24 (22%) died before completing PRRT, 10 (9%) had disease progression, and 7 (7%) had general deterioration. The clinical characteristics of the patients included in the training dataset (10) and in the test dataset of the present study are summarized in Table 1.
Patients’ Demographic and Clinical Data
SPECT/CT data were used to calculate the cumulative radiation ADs for 167 kidneys (right and left), 170 livers, and 150 spleens. Five kidney pairs, 2 livers, and 20 spleens were excluded because of missing archived data, and 20 patients underwent splenectomy. Additional measurements were performed in the bone marrow of 27 patients (145 were excluded because of inappropriate timing of sampling) and 311 tumors using both standard MTP dosimetry and the STP MLR model.
This study was approved by the Institutional Review Board in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The requirement to obtain informed consent was waived.
PRRT Therapy
Infusion of 1.5 L of amino acid solution started at least 30 min before radiopharmaceutical administration and continued for 5–6 h. 177Lu-DOTA-octreotate (Isorad Ltd., Soreq Nuclear Research Center [396 therapy cycles]; S.R.Y Medical Services Ltd. [81 cycles]) was coadministered intravenously over 30 min (11). The mean injected activity per treatment cycle was 7.2 ± 0.7 MBq. The median cumulative activity per patient was 21.9 GBq (range, 5.7–37.5 GBq). The interval between treatment cycles was 5–28 wk (median, 7 wk).
Posttreatment Imaging
SPECT/CT studies of the abdomen, including kidneys, liver, and spleen, were acquired after each cycle of treatment, as previously described (10,12). When necessary for extraabdominal tumor sites, an additional field of view was acquired.
Acquisition parameters and camera calibration were previously described (10). Briefly, studies were acquired either on an Infinia SPECT/CT (GE Healthcare) (n = 12, February to September 2012) or on a Discovery NM/CT 670 (GE Healthcare) (n = 147) with a 20% energy window around the main 208-keV photopeak of 177Lu and medium-energy general-purpose collimators.
Image Reconstruction and Analysis
SPECT images were reconstructed with the ordered-subsets expectation maximization algorithm (2 iterations, 10 subsets), CT attenuation correction, scatter correction, and resolution recovery (for blurring) and were processed using the Dosimetry Toolkit (GE Healthcare) software on the Xeleris 3.0 workstation (GE Healthcare) as previously described (12). Volumes of interest were placed over the entire healthy organs of interest and over tumor sites.
Standard MTP Dosimetry Calculation
Of the 172 PRRTs with MTP dosimetry, 162 (94%) were performed using 3 time points, and in 10 therapies 2-time-point dosimetry was performed. We previously demonstrated that ADs obtained using a 2-time-point dosimetry protocol (24 and 168 h) show mean relative differences lower than 1.0% compared with the European Association of Nuclear Medicine/MIRD protocol (10).
MTP ADs were computed using an in-house interactive data language code, taking as input the output file of the Dosimetry Toolkit, including the volume and activity concentrations in each volume of interest at every time point. The code performs monoexponential curve fitting from MTPs (2 or 3) after the first cycle and from an STP for the following cycles, assuming an unchanged effective half-life between cycles (13,14), and calculates residence times in the different organs and tumors. For organs, the MIRD formalism (15) was used for AD calculation. For tumors, only self-ADs are considered (16). Briefly, the ADs (mGy) were obtained by multiplying the tumor radioactivity concentration residence time ([MBq ⋅ s]/[MBq ⋅ kg]) by the dose concentration factor (0.0236 [mGy ⋅ kg]/[MBq ⋅ s]) and by the administered activity (MBq). For bone marrow, AD blood samples were drawn at a t1 of 24 h and a t3 of 168 h after the first cycle and at t1 after subsequent cycles. Blood activity concentrations were measured using a NaI(Tl) well γ-counter (Wizard 1480 3″; Perkin Elmer). The blood activity concentration was fitted by a monoexponential curve and integrated to infinity to estimate the self-AD to the bone marrow, assuming that the activity concentration in the latter is similar to blood (17).
STP Dosimetry Calculation
Organ and tumor radiation ADs were estimated from a single SPECT/CT study using the MLR model previously developed (10). This model takes 2 independent variables as input (time of imaging after treatment and 177Lu-DOTATATE activity concentration in a given organ or tumor) and predicts the corresponding ADs for solid organs, bone marrow, and tumors. When comparing cumulative kidney dosimetry results obtained using the MTP model and the STP MLR model, the best agreement was achieved using a single SPECT/CT study acquired at a t3 of 168 h after the first therapy cycle and at a t1 of 24 h after the following ones (10). These same time points were used in the present study to predict the AD by solid organs, tumors, and bone marrow.
Solid Organs and Tumors
Radiation ADs by the kidneys, liver, spleen, and tumors (rk) were predicted using the following model with a single SPECT/CT study: Eq. 1with D(rk) being the AD by rk (mGy), ts being the time of imaging (h), ak(ts) being the activity concentration in rk at time ts (MBq/cm3) and α0,k (ln[kg⋅mGy/MBq]), α1,k having no units, and α2,k (s−1) being the regression coefficients at ts.
Eq. 1with D(rk) being the AD by rk (mGy), ts being the time of imaging (h), ak(ts) being the activity concentration in rk at time ts (MBq/cm3) and α0,k (ln[kg⋅mGy/MBq]), α1,k having no units, and α2,k (s−1) being the regression coefficients at ts.
Bone Marrow
The largest contribution to the AD is derived from the self-AD conveyed by the blood, followed by the cross-dose from the remainder of the body (18). Therefore, the model takes as input the activity concentration in blood and in the remainder of the body (MBq/cm3) at time ts: ablood(ts) and aRM(ts), respectively.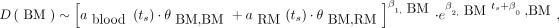 Eq. 2where θBM,BM and θBM,RM are in kg⋅mGy/MBq⋅s; β0,k (ln(s)), β1,k having no units, and β2,k (s−1) being the regression coefficients of the bone marrow (BM) model at time ts.
Eq. 2where θBM,BM and θBM,RM are in kg⋅mGy/MBq⋅s; β0,k (ln(s)), β1,k having no units, and β2,k (s−1) being the regression coefficients of the bone marrow (BM) model at time ts.
Equations 1 and 2 were used for prediction of the radiation ADs by 167 kidneys (462 therapies), 150 spleens (412 therapies), 170 livers (467 therapies), 27 bone marrows (67 therapies), and 311 tumors using a ts of 168 h for the first therapy cycles (n = 172) and a ts of 24 h for the following ones (n = 305). Tables 2 and 3 summarize the regression coefficients used for solid organs, bone marrow, and tumors.
Regression Coefficients of STP MLR Model in Solid Organs and Tumors
Regression Coefficients of STP MLR Model in Bone Marrow
Patient Management from Expected Cumulative Kidney Radiation AD
After each therapy cycle number n, the expected cumulative kidney AD after the following cycle (n + 1) was defined by adding the mean ADs of the previous n therapy cycles to the cumulative AD of the n therapies. When the expected cumulative AD exceeded 25 Gy ± 5%, PRRT was stopped, unless decided otherwise by a multidisciplinary team. Management decisions based on STP MLR calculation were compared with decisions based on MTP dosimetry.
Statistical Analysis
Patients were separated into 4 independent groups according to the number of cycles administered (group 1, 1 cycle [32 patients]; group 2, 2 cycles [36 patients]; group 3, 3 cycles [45 patients]; and group 4, 4 cycles [57 patients]).
Differences between cumulative ADs obtained with MTP and STP MLR-based protocols were assessed with Bland–Altman analysis for each patient group. Median relative differences and range were also calculated. The Pearson r correlation coefficient and the angular coefficient a (slope of the linear regression line) were used for correlation between methods.
To test the hypothesis that patient management based on an STP protocol does not differ from that based on an MTP protocol in at least 90% of the therapies, an exact 1-tailed binomial test was performed separately on each group. A Fisher χ2 test for combined probabilities (19) was performed to combine the P values from the different groups into a single composite P value. A Fisher P value lower than 0.05 was considered statistically significant. For the 2 patients who received 5 cycles, only mean ± SD was calculated, and patient management was not evaluated. SPSS Statistics (IBM Corp., Version 29.0) for Microsoft Windows was used for the analysis.
RESULTS
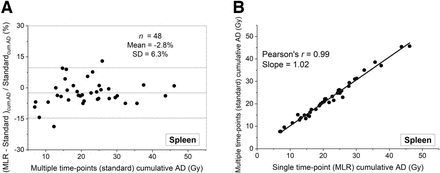
Relative differences and correlations between the cumulative ADs calculated using our standard MTP model and the STP MLR model are respectively shown in Figures 1, 2, 3, and 4 for kidneys, liver, spleen, and tumor sites for patients who received 4 cycles of treatment (group 4). For these patients, mean relative differences of 0.8% ± 8.0%, −7.7% ± 4.8%, 0.03% ± 11.4%, −2.8% ± 6.3%, and −2.1% ± 18.4% were obtained for 56 kidneys, 5 bone marrows, 57 livers, 48 spleens, and 101 tumors, respectively (Pearson r = 0.99 for all). For other patients, similar mean relative differences were obtained. Table 4 summarizes the mean relative differences, median, range, and angular coefficients obtained between both methodologies for organ and tumor cumulative ADs for patients who received 1–5 therapy cycles (Pearson r = 0.99 for all).
Bland–Altman (A) and correlation (B) plots between cumulative kidney ADs after 4 PRRT cycles calculated with STP and MTP protocols.
Bland–Altman (A) and correlation (B) plots between cumulative liver ADs after 4 PRRT cycles calculated with STP and MTP protocols.
Bland–Altman (A) and correlation (B) plots between cumulative spleen ADs after 4 PRRT cycles calculated with STP and MTP protocols.
Bland–Altman (A) and correlation (B) plots between cumulative tumor ADs after 4 PRRT cycles calculated with STP and MTP protocols.
Differences Between STP and Our MTP Calculations
Effective half-lives of 53 ± 10 h (range, 36–100 h), 73 ± 13 h (range, 35–109 h), 72 ± 12 h (range, 41–112 h), and 84 ± 26 h (range, 24–159 h) were obtained with the standard MTP protocol for kidneys, liver, spleen, and tumors, respectively. The regression coefficient α2,k (Eq. 1; Table 2) corresponds to the effective decay constant λk for a given organ or tumor k (10). Comparing the STP model effective half-life ln(2)/α2,k with the effective half-life obtained from our MTP protocol, we obtained similar values of 62, 77, 73, and 81 h with a ts of 168 h for kidneys, liver, spleen, and tumors, respectively.
Patient management based on the expected kidney cumulative ADs after the next treatment, calculated with the STP MLR model, was similar to that for the MTP model in 170 of 172 PRRTs. In 1.2% (2/172) of the PRRT therapies, there were different management decisions, including 1 of 32 patients in group 1 (3.1%) and 1 of 36 in group 2 (2.7%). The agreement was well over 90%, but the differences were not statistically significant at the 0.05 level (0.156 and 0.113, respectively). For groups 3 (n = 45) and 4 (n = 57), there was no difference in management (P = 0.009 and 0.002, respectively). The Fisher χ2 test for combined probabilities produced a composite P value of 0.0003 for the 4 groups.
DISCUSSION
We previously described an MLR model that predicted radiation ADs by organs and tumors from a single SPECT/CT study acquired 1 wk after the first post-PRRT cycle and 1 d after subsequent cycles, in 32 consecutive neuroendocrine neoplasm patients (10). In the present study, the model was evaluated in 159 patients, 172 therapies, and 477 cycles. To the best of our knowledge, this was the first study assessing an STP model after PRRT in a large patient cohort. When the STP MLR model was used, patient management decisions differed from those made using the MTP model in only 2 of 172 therapies (1.2%). A composite Fisher P value of 0.03% for the groups of patients who underwent between 1 and 4 therapy cycles was obtained. Of note, the independent P values for groups 1 and 2 (each with 1 patient mismanaged) were expected to be higher than 0.05 in view of the relatively low number of patients in these groups. However, both mismanaged patients had high kidney ADs after the first PRRT, underestimated by the STP model (11.8 and 10 Gy vs. 14.2 and 14.4 Gy with our standard MTP, respectively). The expected cumulative ADs exceeded the 25-Gy threshold after the second therapy cycle with the standard MTP model and after the third cycle with the STP model. The mean kidney AD with our MTP model in the present study was 5.2 Gy. Patients with high predicted kidney ADs after the first PRRT cycle should be managed carefully, and the MTP methodology should be used for the remaining treatment cycles. Recent studies reported cumulative ADs of as high as 40 Gy (6,8,20,21) with no renal toxicity. Therefore, the STP model with the kidney threshold set at 25 Gy is not expected to lead to significant safety problems even for the 1% of patients managed differently.
There were 3 outlier cumulative kidney ADs, underestimated by the STP MLR model. In 2 patients, PRRT was stopped after a single treatment cycle because of deterioration in the patients’ clinical status. No safety issues are expected in this case. The last patient presented a high kidney effective half-life of 89 h, likely due to obstructive uropathy, which may have caused the relatively large difference in the cumulative AD obtained from both methods. However, such cases are rare.
Hänscheid et al. (22) have previously demonstrated in 29 patients that a single posttreatment SPECT/CT study performed 4 d after PRRT provides a reliable time-integrated activity estimation (self-AD), with median errors of 5%, 6%, 8%, and 6% for kidneys, liver, spleen, and lesions, respectively. In the present study, including 172 PRRTs, median errors in cumulative AD estimates were significantly lower (kidneys, 0.2%; liver, 1.8%; spleen, −2.5%; and tumors, −0.9%). Jackson et al. (23) estimated in 29 patients time-integrated activities from tissue-specific dose conversion factors obtained from the normalization of existing time–activity curves to a single measurement. Recently, Devasia et al. (24) and Hardiansyah et al. (25) estimated in 8 patients the radiation ADs from an STP model using a physiologically based pharmacokinetic model and a nonlinear mixed effect model.
The present study showed that our STP MLR model produces similar dosimetry results and persistent patient management decisions compared with MTP dosimetry. It does not necessarily prove the accuracy of the dosimetry approach or its precision for preventing toxicity. This STP model could potentially be incorporated into clinical trials to evaluate whether safety can be estimated from a single posttreatment imaging study. However, in view of the relatively high SD obtained in the Bland–Altman analysis, it may present challenges for prediction of toxicity at the individual level.
A limitation of the present study is that our MTP protocol includes multiple SPECT/CT studies only after the first therapy cycle and not after each treatment. The model needs to be further tested with MTP dosimetry after each treatment cycle. In addition, 10 of 172 (6%) dosimetry calculations were performed with 2-time-point dosimetry, compared with 162 therapies (94%) with 3 time points. We previously demonstrated mean relative differences in ADs lower than 1.0% ± 4.0% between the 2- and the 3-time-point protocols (26). Although the difference in the cumulative AD obtained in the present study with STP was somewhat higher (−3.0% to 1.5%), it had the same order of magnitude.
CONCLUSION
The present study, performed on a large cohort of 159 patients, showed that dosimetry results derived from a single post-PRRT SPECT/CT study were similar to our standard MTP protocol, with a 1.2% difference in management decisions. STP dosimetry is feasible and can be used with confidence, avoiding the use of laborious software, simplifying calculations, improving patient comfort, and optimizing departmental workflow and productivity.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can radiation ADs by organs and tumors after PRRT be estimated from a single SPECT/CT study?
PERTINENT FINDINGS: Quantitative 177Lu-DOTATATE SPECT/CT data from 159 consecutive patients were retrospectively analyzed to test an STP MLR model predicting the radiation AD from a single posttherapy SPECT/CT study in a large patient group. Cumulative ADs had a mean relative difference from the standard MTP of 0.8% ± 8.0%, −7.7% ± 4.8%, 0.0% ± 11.4%, −2.8% ± 6.3%, and −2.1% ± 18.4% for kidneys, bone marrow, liver, spleen, and tumors, respectively, for patients who underwent 4 therapy cycles. Similar results were obtained with fewer therapy cycles. Differences in management decisions between our standard protocol and the STP model occurred in 1.2% (2/172) of the therapies.
IMPLICATIONS FOR PATIENT CARE: Dosimetry calculations using our MLR model for AD estimation with a single quantitative SPECT/CT study after PRRT are similar to the results obtained using the standard MTP protocol. The MLR model simplifies the dosimetry process, reduces scanner and technician time, and shortens the AD calculation process for the medical physicist. It may optimize departmental workflow and productivity and improve patient comfort.
ACKNOWLEDGMENTS
We thank Prof. Ora Israel for her critical review of the manuscript and useful suggestions, and we thank Prof Norman B. Grover for his statistical suggestions.
Footnotes
Published online Jul. 27, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 23, 2022.
- Revision received May 31, 2023.