Visual Abstract
Abstract
Treatment-induced neuroendocrine prostate cancer (NEPC) is a lethal subtype of castration-resistant prostate cancer. Using the 89Zr-labeled delta-like ligand 3 (DLL3) targeting antibody SC16 (89Zr-desferrioxamine [DFO]-SC16), we have developed a PET agent to noninvasively identify the presence of DLL3-positive NEPC lesions. Methods: Quantitative polymerase chain reaction and immunohistochemistry were used to compare relative levels of androgen receptor (AR)–regulated markers and the NEPC marker DLL3 in a panel of prostate cancer cell lines. PET imaging with 89Zr-DFO-SC16, 68Ga-PSMA-11, and 68Ga-DOTATATE was performed on H660 NEPC–xenografted male nude mice. 89Zr-DFO-SC16 uptake was corroborated by biodistribution studies. Results: In vitro studies demonstrated that H660 NEPC cells are positive for DLL3 and negative for AR, prostate-specific antigen, and prostate-specific membrane antigen (PSMA) at both the transcriptional and the translational levels. PET imaging and biodistribution studies confirmed that 89Zr-DFO-SC16 uptake is restricted to H660 xenografts, with background uptake in non-NEPC lesions (both AR-dependent and AR-independent). Conversely, H660 xenografts cannot be detected with imaging agents targeting PSMA (68Ga-PSMA-11) or somatostatin receptor subtype 2 (68Ga-DOTATATE). Conclusion: These studies demonstrated that H660 NEPC cells selectively express DLL3 on their cell surface and can be noninvasively identified with 89Zr-DFO-SC16.
Androgen receptor (AR) signaling is a critical driver of prostate cancer (PC). Androgen deprivation therapy (ADT) is used in the setting of high-risk, recurrent, or metastatic PC. Although ADT can initially be highly effective, resistance develops in most patients, leading to metastatic castration-resistant PC (mCRPC) and ultimately death. Treatment resistance to ADT in mCRPC arises from multiple mechanisms including AR amplification, AR bypass, and complete AR independence (1). The introduction of more potent AR signaling inhibitors (e.g., abiraterone and enzalutamide) in mCRPC patients has led to an increasing incidence of lesions that may display AR loss or adapt to low androgen levels through activation of alternative pathways (2). AR-independent tumors can be aggressive and can demonstrate markedly elevated proliferation, an enhanced capacity for organ metastasis, and phenotypic heterogeneity with a variable admixture of adenocarcinoma and small cell neuroendocrine phenotypes (2). Treatment-induced neuroendocrine PC (NEPC) has been shown to arise from treatment-induced lineage plasticity and to lead to a highly aggressive and lethal subtype of PC (3). This reprogramming is linked to the reactivation of developmental transcriptional programs and the transcription factor SOX2, which promotes phenotypic plasticity and acquisition of a stemlike phenotype (4). NEPC displays genetic similarities to small cell lung cancer (SCLC), including frequent loss of the tumor suppressor genes TP53 and RB1 (5).
The lineage transcription factor achaete-scute complex homolog 1 (ASCL1) is overexpressed and promotes tumorigenesis in mouse models of SCLC (6). ASCL1 plays a key role in suppressing notch signaling activity through markedly upregulated expression of the inhibitory notch ligand delta-like ligand 3 (DLL3) (7). DLL3 expression in adult tissues is substantially lower and restricted to intracellular compartments such as the Golgi; in contrast, in SCLC tumor cells DLL3 is aberrantly expressed on the cell surface (7). In PC, DLL3 has been shown to be similarly dysregulated in most NEPC samples (76.6%), with minimal to no expression in mCRPC (12.5%), localized PC (0.5%), and normal, unaffected prostate tissue (0%) (8). With no detectable expression of cell surface DLL3 in nonmalignant cells and preferential expression in NEPC lesions, DLL3 may be a target for a biomarker-based method of tumor detection.
SC16 is a humanized monoclonal antibody that selectively binds to both human and murine DLL3 (9). Our previous work with SC16 demonstrated the successful synthesis of 89Zr-radiolabeled SC16 and its use as a PET radiopharmaceutical for noninvasive detection of DLL3 expression in SCLC models (10). PET is a powerful diagnostic tool that can noninvasively visualize and molecularly characterize lesions and aid in optimizing therapy. It has proven to be a successful platform in PC detection and management of AR-dependent lesions. However, the 2018 National Cancer Institute Workshop on Lineage Plasticity and Androgen Receptor-Independent Prostate Cancer identified a “lack of imaging capabilities” as 1 of 2 major knowledge gaps in NEPC management (11). The PET probes thus far developed directly target AR (12) or prostate-specific membrane antigen (PSMA) (13) and may not detect NEPC lesions if they display AR loss or PSMA suppression (14). Therefore, it is imperative to have a non–PSMA-based imaging agent for NEPC detection. To our knowledge, 89Zr-radiolabeled SC16 is the only imaging agent currently in development that has potential to differentiate NEPC from prostate adenocarcinoma by an increase in signal above background. This is advantageous over current PET imaging agents that target AR-dependent PC, as reduced expression of these surface antigens is possible and can increase susceptibility to high false-positives or inconclusive findings when signal approaches the noise floor. Because of the molecular similarities to SCLC and encouraging results from preclinical studies in SCLC models, we evaluated the performance of our 89Zr-radiolabeled SC16 PET imaging agent in the detection of DLL3-expressing NEPC tumors in preclinical mouse models.
MATERIALS AND METHODS
The supplemental materials (available at http://jnm.snmjournals.org) provide information on cell lines, quantitative real-time polymerase chain reaction, antibody functionalization and radiolabeling (15), in vitro binding assays (16), immunohistochemistry, and generation of subcutaneous xenograft models.
Animals and Tumor Models
All animal experiments were approved by the Institutional Animal Care and Use Committee and Research Animal Resource Center at Memorial Sloan Kettering Cancer Center.
Biodistribution Studies
Biodistribution studies were performed by killing mice bearing subcutaneous H660 or DU145 tumors at chosen time points to evaluate uptake of the radiotracers. To evaluate nonspecific uptake in the DLL3-positive tumor, mice bearing subcutaneous H660 xenografts were injected with isotype-matched IgG 89Zr-desferrioxamine (DFO)-IgG. The supplemental materials provide further details.
PET Imaging
PET imaging of subcutaneous PC cell line xenograft mouse models was performed on an Inveon small-animal PET/CT instrument (Siemens). The supplemental materials provide further details.
Statistical Analysis
Data are expressed as mean ± SEM. Data for the in vitro cell binding assay were analyzed by unpaired, 2-tailed t testing using Prism software (version 8; GraphPad), with the threshold for statistical significance set at a P value of less than 0.05. To evaluate the blocking study in the subcutaneous xenografts, a 2-way ANOVA test using Prism software was performed, with the threshold for statistical significance set at a P value of less than 0.05. A correction for multiple comparisons was performed using the Holm–Sidak method to determine statistical significance (α = 0.05).
RESULTS
Evaluation of DLL3 Expression in NEPC
We evaluated messenger RNA and protein expression of AR and SOX2 transcription factors and their downstream targets that have been shown to differentiate adenocarcinoma or neuroendocrine phenotype, respectively, in established PC cell lines using quantitative real-time polymerase chain reaction and immunohistochemistry. For prostate adenocarcinoma genes, we evaluated FOLH1 (gene encoding for PSMA) and KLK3 (gene encoding for prostate-specific antigen); for neuroendocrine-regulated genes, we examined ASCL1 and DLL3. The panel of PC cell lines included LNCaP (hormone-sensitive prostate adenocarcinoma, AR-positive/neuroendocrine-negative), DU145 (androgen-independent PC, AR-negative/neuroendocrine-negative), PC3 (androgen-independent PC, AR-negative/neuroendocrine-negative), and H660 (NEPC, AR-negative/neuroendocrine-positive). H660 expressed the highest of SOX2, which encodes a transcription factor that promotes lineage plasticity, facilitates histologic transformation, and has been associated with resistance to AR-targeted therapies (5,17). PC3, LNCaP, and DU145 all demonstrated significantly lower levels of DLL3. H660 cells showed ASCL1 expression, encoding a key transcription regulator of notch signaling proteins, including DLL3 (Fig. 1A). At the protein level, using immunohistochemistry, we could detect DLL3 expression only in the H660 line, not in the other PC lines (Fig. 1B). Verification of the NEPC lineage of H660 was confirmed by the presence of the clinically validated neuroendocrine marker synaptophysin (SYP), which was absent in all LNCaP, PC3, and DU145 cell lines (Fig. 1B). When transcript levels of AR, KLK3, and FOLH1 were evaluated, only LNCaP cells showed expression, whereas H660, PC3, and DU145 had minimal detectable transcripts (Supplemental Fig. 1A). Immunohistochemistry provided similar results at the translational level, demonstrating intense staining of AR and PSMA in LNCaP cells, with no detectable expression seen in H660, PC3, or DU145 (Supplemental Fig. 1B).
DLL3 is uniquely expressed in H660 cells at transcriptional and translational level. (A) Real-time polymerase chain reaction shows expression patterns of DLL3, ASCL1, and SOX2 genes in H660, DU145, PC3, and LNCaP cells compared with A549-negative control (not shown). (B) Representative immunohistochemistry images of H660 (DLL3-positive/SYP-positive) DU145 (DLL3-negative/SYP-negative), LNCaP (DLL3-negative/SYP-negative), and PC3 (DLL3-negative/SYP-negative) tumor cell sections for DLL3 and SYP. ND = not detected.
We then analyzed the PC transcriptomic atlas dataset (18), an extensive transcriptome databank comprising 1,321 clinical specimens from 38 PC cohorts. We observed higher expression of DLL3 in mCRPC than in primary PC (P < 0.001 mCRPC vs. primary; Supplemental Fig. 2A). Moreover, in the cohort of Ross-Adams et al. (19) (259 men with PC in Cambridge discovery cohort and Stockholm validation cohort), we found that high DLL3 expression was associated with shorter biochemical recurrence-free survival (Supplemental Fig. 2B). Together, these data indicate that DLL3 messenger RNA expression may be associated with a poor prognosis for PC patients. This hypothesis will require further testing in a larger cohort to confirm the prognostic significance of DLL3 messenger RNA expression in PC.
We next compared the expression levels of DLL3 in H660 and H82 tumor cells. H82 was derived from the pleural fluid of a patient with SCLC and H660 derived from the lymph node of an NEPC patient. Both cell lines displayed a neuroendocrine phenotype and aberrant DLL3 trafficking to the cell surface. Despite low expression of DLL3 on H82 cells, PET imaging successfully delineated H82 tumor in mouse models (10). Immunohistochemistry demonstrated an even lower abundance of DLL3 expression in the H660 tumor cells than in the H82 tumor cells (Supplemental Fig. 3). However, we still envision that DLL3 PET imaging would be feasible because of the absence of DLL3 expression on the cell surface of nonmalignant cells.
89Zr-DFO-SC16 Characterization and Its Detection of Cell Surface DLL3 Expression
The DLL3-targeting SC16 antibody was functionalized through non–site-specific conjugation to the free lysine moieties available on the monoclonal antibody with the siderophore-derived DFO chelator (Supplemental Fig. 4). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry revealed that the ratio of chelator to monoclonal antibody was 0.44, which implies that, on average, half of the antibody molecules were functionalized with DFO covalently (Supplemental Fig. 5).
Radiolabeling of the DFO-SC16 conjugate provided 89Zr-DFO-SC16 in high radiochemical yield (>99%) (Supplemental Fig. 6A) and high specific activity (370 MBq/mg). The radioimmunoconjugate demonstrated at least 92% stability when incubated in human serum at 37°C for 5 d (Supplemental Fig. 6B). The target-binding fraction of the radioimmunoconjugate—the fraction of the antibody that retains its binding to its target after modification—was more than 90% for 89Zr-DFO-SC16 toward DLL3 (Fig. 2A). 89Zr-DFO-SC16 binding to DLL3 could be blocked in the presence of a 5,000-fold excess of unlabeled SC16. In vitro cell binding assays revealed 89Zr-DFO-SC16 to be bound to DLL3 expressed on the cell surface of H660 cells, with no binding observed in the DLL3-negative cell lines LNCaP, DU145, or PC3 (Fig. 2B). Binding of 89Zr-DFO-SC16 to H660 cells could be blocked in the presence of a 1,000-fold excess of unlabeled SC16. A saturation binding assay with H660 cells revealed a dissociation constant of less than 1 nM and a maximum specific binding of 86.3 fM/106 cells for 89Zr-DFO-SC16 (Supplemental Fig. 6C). These studies demonstrated that the immunoreactivity of SC16 is retained after DFO conjugation and radiolabeling with 89Zr.
DLL3 is expressed on cell surface of H660 tumor cells that can be targeted by 89Zr-DFO-SC16. (A) 89Zr-DFO-SC16 shows high and specific binding to Ni-NTA beads coated with His-tagged DLL3. Minimum nonspecific binding is observed (control). Specificity of binding to His-tagged DLL3 was shown in presence of 5,000-fold excess of unlabeled SC16. (B) In vitro cell binding data on 89Zr-DFO-SC16 binding to H660 cells confirms DLL3 expression on cell surface that can be targeted using our anti-DLL3 monoclonal antibody SC16. Minimal binding of 89Zr-DFO-SC16 is observed to DLL3-negative DU145, PC3, and LNCaP cells. Specificity of binding to H660 cells was shown in presence of 1,000-fold excess of unlabeled SC16. ****P < 0.0001. NSB = nonspecific binding.
PET Imaging and Biodistribution of DLL3 Expression with 89Zr-DFO-SC16 in Subcutaneous Xenograft Models
PET imaging with 89Zr-DFO-SC16 was performed on male nude mice bearing H660 (DLL3-positive, AR-negative/neuroendocrine-positive) or DU145 (DLL3-negative, AR-negative/neuroendocrine-negative) subcutaneous xenografts. PET imaging with 89Zr-DFO-SC16 showed clear delineation of H660 tumor xenografts 120 h after administration of the radioimmunoconjugate (Fig. 3A). Increasing uptake in the H660-bearing mice could be observed in the tumors from the time of injection to 120 h after injection. As expected, because of lack of DLL3 expression, minimal uptake of 89Zr-DFO-SC16 was observed in DU145-bearing mice, with tumor uptake remaining constant over the 120-h imaging time course (Fig. 3B). Low and nonspecific uptake was observed in H660 tumor xenografts 120 h after injection of isotype-matched IgG (89Zr-DFO-IgG) (Supplemental Figs. 7A–7C).
DLL3-expressing subcutaneous H660 tumors could be imaged with 89Zr-DFO-SC16 in vivo. (A) PET images of 89Zr-DFO-SC16 in athymic nude male mouse bearing subcutaneous H660 tumor xenografted on left shoulder. PET imaging was performed at 24-h intervals up to 120 h after injection of 89Zr-DFO-SC16. (B) PET images of 89Zr-DFO-SC16 in athymic nude male mouse bearing subcutaneous DU145 tumor xenografted on left shoulder. PET imaging was performed at 24-h intervals up to 120 h after injection of 89Zr-DFO-SC16. Images represent maximum-intensity projection and transverse planar images at designated time points after injection of radiotracer. MIP = maximum-intensity projection.
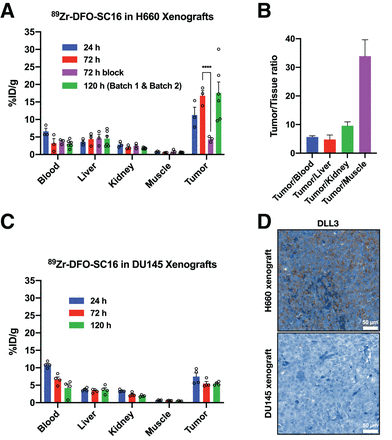
Biodistribution data at 24, 72, and 120 h after intravenous administration confirmed a progressive increase in H660 tumor uptake over time, reaching 17.50 ± 3.19 percentage injected dose per gram (%ID/g) at 120 h (Fig. 4A; Supplemental Fig. 8). As expected, a concurrent decrease in blood-pool activity was observed (24 h, 6.60 ± 0.90 %ID/g; 72 h, 3.16 ± 1.37 %ID/g; 120 h, 3.07 ± 0.45 %ID/g) as the tumor activity increased over time. Because of heterogeneous uptake of the radioimmunoconjugate between the H660 tumors, 2 independent cohorts of 3 mice each were evaluated at 120 h. Linear regression analysis displayed a positive correlation between time after intravenous administration and %ID/g in H660 tumors across both independent cohorts, with tumor uptake increasing over time (P = 0.00971; Supplemental Fig. 9). Uptake of the radioimmunoconjugate could be blocked by coinjection of a 50-fold excess of unlabeled SC16 antibody, confirming the specificity of the radioimmunoconjugate for the H660 tumor (Fig. 4A; Supplemental Fig. 10). At 120 h after injection, high tumor-to-background contrast ratios were observed (Fig. 4B), with a tumor-to-muscle ratio of 33.91 ± 5.73. In contrast, 89Zr-DFO-SC16 uptake in the DU145 (DLL3-negative) tumors was low and nonspecific, at 5.47 ± 0.25 %ID/g 120 h after intravenous administration, consistent with an in vivo enhanced permeability and retention effect (Fig. 4C; Supplemental Fig. 11). Comparing tumor uptake at each time point in the H660 and DU145 tumors demonstrated the specificity and selectivity of 89Zr-DFO-SC16 for DLL3 (Supplemental Fig. 12A). Mean uptake in H660 tumors increased over time and was statistically significant when compared with mean uptake in DU145 tumors at 72 and 120 h after injection. Tumor-to-muscle ratios also increased over time in the H660 tumors and were statistically significant at 72 and 120 h after injection when compared with DU145 tumors, in which this ratio remained relatively constant at all time points (Supplemental Fig. 12B). Considering potential clinical implications, taken together these data suggest that patients could be effectively imaged in a wide time window (72–120 h). Immunohistochemistry on resected H660 subcutaneous xenografts confirmed retained expression of DLL3 and SYP, which were absent in resected DU145 subcutaneous xenografts (Fig. 4D; Supplemental Fig. 13 shows SYP immunohistochemistry and corresponding hematoxylin- and eosin-stained slides).
Biodistribution of 89Zr-DFO-SC16 in subcutaneous xenograft model of NEPC. (A) Select organ biodistribution data at 24, 72, and 120 h after intravenous injection of 89Zr-DFO-SC16 in athymic nude mice bearing subcutaneous H660 tumors. Bars at 24 and 72 h represent data obtained from batch 1 (n = 3). Bars at 120 h represent data obtained from batches 1 and 2 (n = 6). Supplemental materials provide details on the 2 different cohorts used in biodistribution study. H660 tumor uptake could be blocked at 72 h after injection of 89Zr-DFO-SC16 with 50-fold excess of unlabeled SC16 antibody. (B) Tumor-to-background contrast ratios from uptake of 89Zr-DFO-SC16 in H660 xenograft–bearing mice. (C) Select organ biodistribution at 24, 72, and 120 h after injection of 89Zr-DFO-SC16 in athymic nude mice bearing subcutaneous DU145 tumors. (D) Representative immunohistochemistry images of H660 and DU145 subcutaneous tumor xenografts for DLL3. ****P < 0.0001.
NEPC Is Detectable Only by DLL3 Imaging, Not by PSMA or Somatostatin Imaging
To demonstrate the dichotomy between AR-positive prostate adenocarcinoma and AR-negative NEPC, male nude mice were subcutaneously xenografted with PSMA-positive/DLL3-negative LNCaP tumors and PSMA-negative/DLL3-positive H660 tumors on opposite flanks and imaged by DLL3- and PSMA-based PET. The dually tumor-bearing mice were imaged 1 h after administration of 68Ga-PSMA-11, a PSMA-targeted PET agent. The radiotracer accumulated exclusively in the LNCaP tumor and could be successfully imaged with high contrast (Fig. 5). The NEPC model H660 does not express AR or PSMA, and H660 tumors could not be detected through PSMA-based imaging. Two days later, the same cohort of mice was administered 89Zr-DFO-SC16 and imaged 5 d after administration. The radiotracer selectively bound to the H660 tumor but not to LNCaP and finally allowed for site-specific identification of the NEPC lesion.
68Ga-PSMA-11 cannot image NEPC lesions in vivo. Shown are PET images of 68Ga-PSMA-11 (1 h after injection) or 89Zr-DFO-SC16 (120 h after injection) in male athymic nude mouse bearing H660 and LNCaP tumors xenografted on left and right shoulders, respectively. MIP = maximum-intensity projection.
Somatostatin receptor (SSTR) imaging has been widely used as a method to detect the presence of gastroenteropancreatic neuroendocrine neoplasms, including the Food and Drug Administration–approved agent 68Ga-DOTATATE. 68Ga-DOTATATE is a peptide-based PET imaging agent that demonstrates high affinity for the SSTR subtype 2 (SSTR2) and has been tested in NEPC patients with inconclusive results (20). As a comparator, we sought to image tumor-bearing mice by 68Ga-DOTATATE. We first analyzed SSTR2 messenger RNA expression in H660 and LNCaP cells. We could not detect significantly upregulated SSTR2 expression in H660 cells relative to LNCaP cells (Supplemental Fig. 14A). Shifting to in vivo analysis, we conducted an imaging study with 68Ga-DOTATATE on H660-bearing male nude mice. No accumulation of the radiotracer was observed in the tumor 1 h after administration, demonstrating an inability to detect this NEPC tumor through SSTR imaging (Supplemental Fig. 14B).
DISCUSSION
NEPC is a lethal disease, and detecting the presence of NEPC lesions noninvasively remains challenging. One of the barriers to understanding the biology of mCRPC is the inaccessibility of the tissue and consequent undersampling of disease heterogeneity. Biopsies (the gold standard in terms of phenotypic characterization with immunostaining) and genotypic characterization with next-generation sequencing techniques describe only a single lesion rather than the full diversity of the disease. Furthermore, bone biopsies are difficult because tumors are frequently embedded deep within the densely sclerotic bone, a result of exuberant hydroxyapatite deposition from tumoral stimulation of osteoblasts. Circulating tumor cells and cell-free DNA can be used to characterize the biology of mCRPC and can reflect posttreatment changes in tumor burden but cannot localize the emergence of resistant disease on a lesional level. A noninvasive, whole-body method for detecting the emergence of NEPC will overcome these limitations of standard methods of tumor analysis, allow for identification and characterization of lesions in real time, and facilitate earlier intervention to improve patient survival. Here, we confirmed that the NEPC cell line H660 is devoid of the commonly targeted prostate biomarkers AR, PSMA, and prostate-specific antigen but selectively expresses DLL3 on the cell surface. However, this is an idealized preclinical model, and a varied range of prostate biomarker expression may still be observed in patients with NEPC lesions. In our previous work using the SCLC tumor model H82, we successfully showed that, though expression of DLL3 is less abundant than other commonly imaged tumor-associated antigens (e.g., human epidermal growth factor receptor 2, human epidermal growth factor receptor, and PSMA), the highly tumor-restricted cell surface expression of DLL3 on SCLC allows for successful PET imaging (10). Although the expression levels of DLL3 observed in the H660 NEPC tumor cells were lower than in the H82 SCLC tumor cells, PET imaging was still feasible because of the absence of detectable cell surface DLL3 in nonmalignant cells. We demonstrated that 89Zr-DFO-SC16 shows high specific uptake in H660 (DLL3-positive) tumors through biodistribution studies and PET imaging. PET imaging in a dual tumor model with H660 (DLL3-positive/PSMA-negative) and LNCaP (DLL3-negative/PSMA-positive) tumor cells demonstrated an inability to detect PSMA-negative NEPC lesions with other commonly used imaging approaches. Neuroendocrine differentiation in prostate adenocarcinoma lesions due to selective treatment pressure can lead to the activation of different pathways, including the possibility of low AR and low AR-pathway signaling, which may result in the inability to detect such lesions with 68Ga-PSMA-11 (14) or other AR-targeted tracers such as 18F-16β-fluoro-5α-dihydrotestosterone (12,21). Therefore, using 68Ga-PSMA-11 only, NEPC lesions may not be detected.
DLL3 imaging can provide patient stratification and suggests a rationale for DLL3-targeted therapeutic approaches. Four DLL3-specific therapies have been tested clinically: the antibody–drug conjugate rovalpituzumab tesirine, the bispecific T cell engager AMG757, the chimeric antigen receptor T cell AMG119, and the trispecific T-cell engager HPN328. Rovalpituzumab tesirine therapy demonstrated minimal responses in SCLC and other neuroendocrine carcinomas (9/69; 13%) (22), whereas AMG757 (NCT03319940), AMG119 (NCT03392064), and HPN328 (NCT04471727) are currently being tested in SCLC patients in first-in-humans studies. One avenue yet to be explored could use a targeted radionuclide therapy with 177Lu in patients with detected DLL3-positive PC lesions via 89Zr-DFO-SC16 PET. Recent work by our lab has demonstrated the exceptional efficacy of 177Lu-labeled-SC16 for the treatment of SCLC in preclinical models (23).
Lastly, we demonstrated an inability to target SSTRs in a subcutaneous NEPC model. Reports on the presence of SSTRs in NEPC have been contradictory and inconclusive, with some studies suggesting that SSTR2 expression is upregulated (24,25) whereas others have shown low or absent expression (26,27). Here, we showed that H660 tumor cells do not express SSTRs and therefore cannot be imaged in vivo through somatostatin-based receptor PET imaging.
Although DLL3 appears to be a potential target for PET imaging in NEPC lesions, the biologic consequences of PC treatments on preexisting NEPC lesions are not understood. PC lesions in patients may have distinct adenocarcinoma, neuroendocrine, or mixed phenotypes. Therefore, even with detection of NEPC, patients may continue ADT. ADT influences the expression of prostate-specific antigen and PSMA and blocks AR-targeting agents and therefore influences the outcomes of PET imaging studies that are specific for these targets (28–30). There is no clear understanding of the biologic consequence of ADT on neuroendocrine cells or on the expression of DLL3. Likewise, it has been shown that the phosphatidylinositol 3-kinase pathway is activated in ADT-resistant mCRPC and influences the expression of neuroendocrine markers (31,32). Two phosphatidylinositol 3-kinase inhibitors, everolimus (NCT00976755) and BKM-120 (NCT01385293), are in clinical trials for mCRPC. Further studies to evaluate the potential influence of PC treatments on NEPC lesions and DLL3 expression are warranted.
Overall, the selective expression of DLL3 in the tumor, with no detectable expression in nonmalignant cells, and the high specificity of tumor uptake of 89Zr-DFO-SC16 suggest the possibility of translating this imaging method into the clinic. With a clinical trial (NCT04199741) currently under way at Memorial Sloan Kettering Cancer Center—the first-in-humans clinical trial of DLL3 PET imaging in patients with SCLC—our data encourage the use of DLL3 PET imaging in patients with NEPC lesions. Ultimately, DLL3-targeted PET imaging might confirm the presence of NEPC lesions through a noninvasive whole-body PET scan, aid patient selection, and improve therapeutic outcomes in NEPC-directed clinical trials.
CONCLUSION
These findings suggest that 89Zr-DFO-SC16 is a noninvasive diagnostic PET agent that can be used as a tool to detect the presence of DLL3-positive NEPC lesions at single-lesion resolution. We hope these findings will improve on the current approach of biopsy-based and genotypic characterization in identifying these patients.
DISCLOSURE
The study was supported in part by NIH T32 GM073546 (Joshua Korsen), NIH R35 CA232130-01A1 (Jason Lewis), R35 CA263816 (Charles Rudin), the Geoffrey Beene Cancer Research Center (Jason Lewis, Nagavarakishore Pillarsetty, Charles Rudin, and Yu Chen), and the Department of Defense Idea Award grant W81XWH-19-1-0536 (Nagavarakishore Pillarsetty). The Radiochemistry and Molecular Imaging Probes Core Facility, the Small Animal Imaging Facility, and the Molecular Cytology Core Facility were supported in part by NIH P30 CA08748. Goutam Chakraborty is supported by a Prostate Cancer Foundation Young Investigator Award. Jason Lewis is an associate editor of The Journal of Nuclear Medicine but had no involvement or access to information regarding the peer review of this article. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can DLL3-targeted PET be used as a tool for the noninvasive diagnosis of NEPC?
PERTINENT FINDINGS: DLL3 is expressed exclusively on the cell surface of the H660 NEPC cell line and can be detected with the DLL3-targeting tracer 89Zr-DFO-SC16. In vivo PET imaging studies showed that 89Zr-DFO-SC16 is selective for DLL3-expressing NEPC lesions and cannot be imaged with 68Ga-PSMA-11 or 68Ga-DOTATATE. The selective expression of DLL3 in the tumor and specific tumor uptake of 89Zr-DFO-SC16 suggest potential translation of this imaging method to the clinic.
IMPLICATIONS FOR PATIENT CARE: PET imaging of DLL3 may allow for the identification of NEPC in patients in a benign, noninvasive way early enough to better inform clinical decision making.
ACKNOWLEDGMENTS
We acknowledge the Radiochemistry and Molecular Imaging Probes Core Facility, the Small Animal Imaging Facility, and the Molecular Cytology Core Facility. The graphical abstract and part of Figure 5 were made in Biorender.com.
Footnotes
Published online Jan. 20, 2022.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 3, 2021.
- Revision received January 12, 2022.