Abstract
1319
Objectives: Prostate-specific membrane antigen (PSMA) is highly expressed in the tumor tissue of more than 80% of men with prostate cancer (PC), making it a promising theranostic target. 68Ga-PSMA-R2 is a novel, systemically delivered PSMA-targeted PET/CT radiotracer. We assessed the safety and tolerability of 68Ga-PSMA-R2 in patients with biochemical recurrence (BCR) or metastatic PC (mPC). Preliminary diagnostic value of 68Ga-PSMA-R2 compared with conventional morphological imaging was also assessed.
Methods: PROfind (NCT03490032) was a phase 1/2, first-in-human, open-label, multicenter study enrolling adult men with BCR or mPC. The study comprised: phase 1, patients with BCR; and phase 2, patients with BCR or mPC. For all patients, baseline conventional scans (CT or MRI and/or bone scan) were analyzed. Patients received an intravenous dose of 68Ga-PSMA-R2 (3 MBq/kg; ≥ 150 MBq; ≤ 250 MBq) and underwent safety assessments on the day of injection and at follow-up. In phase 1, serial blood and urine samples were collected up to 6 hours post-injection for pharmacokinetics and dosimetry. Patients in phase 1 underwent whole-body PET/CT scans at 20-30 min, 1, 2 and 3-4 hours post-injection to determine organ and tumor doses, and in phase 2 at 1 and 2 hours post-injection. Dosimetry analysis was performed using OLINDA/EXM v2.0. Potential metastatic lesions were identified by PET/CT (areas showing higher SUVmax than gluteal or thigh muscle) and conventional imaging by two independent, blinded panels of experts. A third panel correlated the identified lesions. The primary endpoint was number of patients with treatment-emergent adverse events (TEAEs). Secondary endpoints included pharmacokinetics, biodistribution, dosimetry and correlation to conventional imaging.
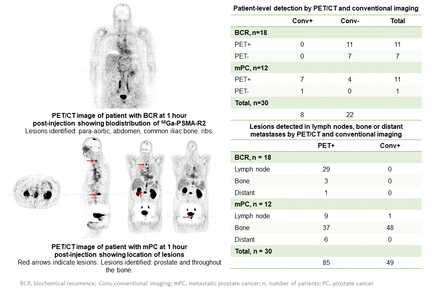
Results: Of 30 patients enrolled, 6 patients with BCR entered phase 1 and 24 patients with BCR or mPC entered phase 2 (n = 12 each). Thirteen TEAEs (n = 7, 23.3%) were reported; 1 was a serious AE (ileus) and there were no deaths. TEAEs occurring in ≥ 5% of patients were fatigue (n = 3, 10.0%) and rash (n = 2, 6.7%). TEAEs were mild or moderate; none were considered related to 68Ga-PSMA-R2 by the investigator. Peak blood concentrations of 68Ga-PSMA-R2 occurred at 5 min post-injection in patients in phase 1, followed by rapid clearance over 6 hours, allowing imaging at 1 hour post-injection. The organ with the highest mean absorbed dose was the urinary bladder (0.1203 ± 0.0517 mGy/MBq). The mean radiation dose for other organs did not exceed 0.10 mGy/MBq, including the kidney (0.0607 ± 0.0191 mGy/MBq). Low doses of radiation were recorded in the salivary (0.0156 ± 0.0032 mGy/MBq) and lacrimal (0.0081 ± 0.0016 mGy/MBq) glands. The mean effective dose was 0.0146 ± 0.0019 mSv/MBq. At 1 hour post-dose, PET/CT imaging detected potential lesions in 11/18 patients with BCR (PSA levels (ng/mL): ≤ 1, n = 5/11; > 1, n = 6/11) and in 11/12 patients with mPC (Figure). Conventional imaging detected potential lesions in 8/12 patients with mPC; none were identified in patients with BCR. One patient with mPC identified as positive by conventional imaging was negative by PET/CT. Percentage agreement at patient level between scans was 46.7% (95% CI: 28.34, 65.67). In patients with BCR, 33 potential lesions were detected by PET/CT but none were detected by conventional imaging. In patients with mPC, 52 potential lesions were detected by PET/CT and 49 by conventional imaging; of these 37 and 48 were bone lesions, respectively (Figure).
Conclusions: 68Ga-PSMA-R2 was well tolerated, with no related AEs, consistent with the safety profiles of PSMA-targeted PET agents. Lesion detectability in patients with BCR and mPC and the low radiation dose absorbed by the salivary and lacrimal glands compared with other PSMA PET agents supports potential therapeutic use of PSMA-R2 by labeling with beta- or alpha-particle emitting radionuclides. Funding: Advanced Accelerator Applications, a Novartis company.