Visual Abstract
Abstract
Prostate-specific membrane antigen (PSMA) and gastrin-releasing peptide receptors are both overexpressed in prostate cancer (PC) but may provide complementary information.68Ga-PSMA-R2 and 68Ga-NeoB (DOTA-p-aminomethylaniline-diglycolic acid-DPhe-Gln-Trp-Ala-Val-Gly-His-NH-CH[CH2-CH(CH3)2]2) are novel PET radiopharmaceuticals that were developed for theranostic use. In this phase II imaging study, we assessed the feasibility, safety, and diagnostic performance of 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI for detection of biochemically recurrent PC. Methods: We prospectively enrolled 27 men with suspected biochemically recurrent PC after initial treatment but noncontributory conventional imaging results (negative or equivocal findings on MRI, CT, and/or bone scan). Participants underwent 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI within 2 wk in noncontrolled order. The SUVmax of putative PC lesions was measured and compared with a composite reference standard (histopathology, follow-up imaging, prostate-specific antigen change). The SUVmax and SUVmean of background organs were measured. Vital signs were recorded before injection of the radiopharmaceuticals and after the scans. Adverse events were recorded up to 72 h after each scan. Results: The prostate-specific antigen level at enrollment was 3.5 ± 3.9 ng/mL (range, 0.3–13.5 ng/mL). 68Ga-NeoB PET/MRI detected 31 lesions in 18 patients (66.7%), whereas 68Ga-PSMA-R2 identified 20 lesions in 15 participants (55.6%). 68Ga-NeoB PET/MRI showed higher sensitivity (85.7% vs. 71.4%), accuracy (88.9% vs. 77.8%), and negative predictive value (66.7% vs. 50.0%) than 68Ga-PSMA-R2, whereas specificity and positive predictive value were equally high (100.0% for both). In 6 patients, 68Ga-NeoB PET/MRI identified 14 lesions that were false-negative on 68Ga-PSMA-R2 PET/MRI. The mean lesion SUVmax was 6.6 ± 3.2 (range, 2.9–13.2) for 68Ga-NeoB and 4.4 ± 1.5 (range, 2.6–8.8) for 68Ga-PSMA-R2 (P = 0.019). Overall lower uptake was noted in tumors and background organs for 68Ga-PSMA-R2. There were no significant changes in vital signs before and after the scans. No adverse events were reported in the 72-h period after scans. Conclusion: 68Ga-NeoB and 68Ga-PSMA-R2 are safe for diagnostic imaging. 68Ga-NeoB PET/MRI showed better diagnostic performance than 68Ga-PSMA-R2. 68Ga-PSMA-R2 showed overall lower uptake, equally in background organs and tumors, and might therefore not be an ideal theranostic compound. Further evaluation in larger cohorts is needed to confirm our preliminary data.
Prostate cancer (PC) remains the most diagnosed cancer in men and the second leading cause of cancer-related death among men in the United States (1,2). Initial treatment with curative intent for localized disease includes radical prostatectomy and radiation therapy. Nonetheless, in up to 53% of patients, biochemically recurrent (BCR) disease will develop within 10 y after definitive treatment (3,4). Early identification and localization of recurrent disease is critical to guide treatment and improve patient outcomes. U.S. and European guidelines (4,5) recommend conventional imaging consisting of CT, MRI, and bone scintigraphy at the time of biochemical recurrence; however, these modalities all bear certain limitations, particularly at low prostate-specific antigen (PSA) levels and in low-volume disease (6,7). Therefore, the most recent National Comprehensive Cancer Network guideline includes prostate-specific membrane antigen (PSMA) PET.
Molecular imaging using radiopharmaceuticals that target tumor-specific cell receptors has revolutionized oncologic imaging. PSMA is overexpressed in 90% of PC cells (8). 68Ga-PSMA-R2 is a novel, urea-based ligand of PSMA that was developed for theranostic use (9). Preliminary results of the phase I/II PROfind trial (NCT03490032) showed a favorable biodistribution with rapid blood clearance (10). Gastrin-releasing peptide receptors (GRPRs) are also overexpressed in PC (11) and BCR PC (12–14). 68Ga-NeoB (DOTA-p-aminomethylaniline-diglycolic acid-DPhe-Gln-Trp-Ala-Val-Gly-His-NH-CH[CH2-CH(CH3)2]2), formerly known as 68Ga-NeoBOMB1, is a DOTA-coupled GRPR antagonist and was also developed for theranostic use. In preclinical and clinical studies, 68Ga-NeoB showed an appropriate pharmacokinetic profile with high receptor affinity, high in vivo stability, and a high tumor-to-background ratio as well as a favorable safety profile (15,16). PSMA- and GRPR-targeting radiotracers have been reported as complementary to each other (14,17,18), however, more studies are needed to understand their respective expression patterns.
In this prospective phase II study we aimed to assess the feasibility, safety, and diagnostic performance of 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI in patients with BCR PC and prior negative or equivocal findings on conventional imaging.
MATERIALS AND METHODS
Participants
Informed oral and written consent was obtained from each participant before enrollment. The participants who were enrolled had noncontributory conventional imaging findings (negative or equivocal findings on CT, MRI, and/or bone scan; Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org) but clinically suspected BCR disease of histopathologically proven PC or PSA persistence after initial treatment (radical prostatectomy or radiation therapy). BCR disease was defined as a PSA level of at least 0.2 ng/mL with a second confirmatory PSA measurement of at least 0.2 ng/mL after radical prostatectomy (19), or a PSA level of at least 2 ng/mL above the nadir after radiation therapy (20). Further inclusion criteria were an age of at least 18 y and a Karnofsky performance status of at least 50. Exclusion criteria were an inability to lie supine for the duration of the scan or metallic implants. Participants were scheduled to undergo either 68Ga-NeoB followed by 68Ga-PSMA-R2 PET/MRI within 2 wk or vice versa. This prospective phase II study was approved by the local institutional review board and registered on ClinicalTrials.gov (NCT03698370). Patients’ vital signs (heart rate, blood pressure, respiratory rate, pulse oximetry) were collected before injection of the radiopharmaceuticals and after completion of the imaging study. Adverse events were recorded for up to 72 h after each scan and were categorized according to the Common Terminology Criteria for Adverse Events, version 5, as part of the safety analysis.
PET/MRI Protocol
Imaging was performed using a 3-tesla time-of-flight–enabled PET/MRI scanner (Signa; GE Healthcare), as previously described (17,21). Simultaneous PET/MRI was acquired from vertex to mid thighs with an acquisition time of 4 min per bed position. Additional dedicated 20-min pelvic images were acquired.
Image Analysis
PET/MRI data were anonymized and then reviewed and analyzed by 2 nuclear medicine physicians independently and in random order. The readers were not aware of any clinical information other than that patients were scanned for BCR PC. Any focal uptake of 68Ga-NeoB or 68Ga-PSMA-R2 in putative sites of disease with an SUVmax above the background level and not associated with a physiologic accumulation was recorded as suggestive of PC. A region of interest was drawn over suspected lesions to measure SUVmax. SUVmax and SUVmean were measured in a volume of interest of 1 cm3 for the blood pool (aortic arch), liver (segment VIII), and background organs bone (femur head) and gluteal muscle, as well as the pancreas (body) for 68Ga-NeoB and parotid gland for 68Ga-PSMA-R2 PET. Tumor-to-background ratios were calculated to quantify uptake in tumors relative to background organs. MRI was used for anatomic and lesion correlation.
Lesion Validation
A composite reference standard was used for lesion validation: histopathology whenever available, subsequent imaging within 2 mo, and posttreatment PSA within 2 mo. A continuous decrease or rise in PSA identified at 2 time points with a minimum interval of 4 wk was considered a treatment response or progressive disease.
68Ga-NeoB or 68Ga-PSMA-R2 PET/MRI was considered true-positive when at least one of the following criteria was met: histopathologic confirmation; progression in number of uptake sites or uptake intensity on follow-up molecular imaging with respective increase in PSA; confirmation on follow-up conventional imaging (CT or MRI); or disappearance or reduction of disease on molecular or conventional imaging after focal or systemic treatment, with a respective decrease in PSA.
68Ga-NeoB or 68Ga-PSMA-R2 PET/MRI was considered true-negative when there was no evidence of disease on follow-up conventional or molecular imaging or when PSA was stable or decreased.
Participants without any composite reference standard correlation were excluded from the analyses.
Statistical Analysis
Statistical analyses were performed using Stata 17.0 (StataCorp LP). Patients’ clinical and imaging characteristics are reported as descriptive statistics, given as mean ± SD, range, and percentage. Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value are given in percentage with 95% CI. A post hoc analysis was performed to stratify the detection rates of 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI by PSA value (<0.5 ng/mL; 0.5 to <1.0 ng/mL; 1.0 to <2.0 ng/mL; 2.0 to <5.0 ng/mL; ≥5.0 ng/mL) and PSA doubling time (<3 mo; 3 to <6 mo; 6 to <9 mo; 9 to <12 mo; ≥12 mo). A 2-sample proportion test was used to compare the SUVmax of positive and negative lesions and to compare the SUVmax and SUVmean of background organs. A P value of less than 0.05 was considered significant.
RESULTS
Patient Characteristics
Twenty-seven men, 67.8 ± 8.8 y old (range, 49.0–82.0 y), were prospectively enrolled. The PSA level at the time of the scan was 3.5 ± 3.9 ng/mL (range, 0.3–13.5 ng/mL). The PSA doubling time was 6.4 ± 5.2 mo (range, 1.2–22.4 mo); 3 participants showed PSA persistence after radical prostatectomy. The image acquisition started at 49.7 ± 7.2 min (range, 43–77 min) after administration of 198.1 ± 25.5 MBq (range, 145.1–245.6 MBq) of 68Ga-NeoB and at 47.0 ± 2.8 min (range, 44–55 min) after administration of 186.1 ± 29.2 MBq (range, 108.4–228.6 MBq) of 68Ga-PSMA-R2. Pelvic images were acquired after a delay of 35.9 ± 5.4 min (range, 21–46 min) for 68Ga-NeoB and 37.4 ± 4.5 min (range, 29–54 min) for 68Ga-PSMA-R2. The PET/MRI scans were performed 11.0 ± 15.2 d (range, 1–72 d) apart. Because the study was conducted during the coronavirus disease 2019 pandemic, the 2-wk interval between scans could not always be maintained. In 4 participants, scans were performed 21, 35, 40, and 72 d apart. The participants’ characteristics are summarized in Table 1.
Patients’ Characteristics
Patients were followed for 24.8 ± 3.9 mo (range, 17.2–31.7 mo) after the PET/MRI scans. Subsequent patient management included radiation therapy in 6 (22%) of the 27 participants; radiation therapy in combination with androgen deprivation therapy in 7 (26%), and androgen deprivation therapy in 8 (30%). Six participants (22%) were under active surveillance.
Safety
There were no significant changes in heart rate, blood pressure, respiratory rate, or pulse oximetry before and after injection of either radiopharmaceutical, nor were there any grade 1 or worse adverse events reported in the 24- to 72-h period after image studies for either radiopharmaceutical.
Lesion Analyses
Lesion validation was follow-up imaging for 18 (63%) of the 27 patients, PSA change in 7 (26%), and histology in 3 (11%).
68Ga-NeoB PET/MRI
68Ga-NeoB PET/MRI identified 31 lesions in 18 (66.7%) of the 27 patients: 1 (3.2%) of these 31 lesions was local recurrence in the prostate, 1 (3.2%) was in the seminal vesicle, 10 (32.3%) were in pelvic lymph nodes, 4 (13%) were in extrapelvic lymph nodes, 14 (45.2%) were in bone, and 1 (3.2%) was a lung metastasis. On a per-lesion level, all were confirmed true-positive using the reference standard (Supplemental Table 2). The mean lesion SUVmax was 6.6 ± 3.2 (range, 2.9–13.2), with a mean tumor-to-background ratio of 11.4 ± 5.1 (range, 4.9–23.2). All SUVs are summarized in Table 2 and Supplemental Table 3. The PSA levels of patients with positive 68Ga-NeoB findings were significantly higher than those of patients with negative 68Ga-NeoB findings (3.9 ± 4.1 ng/mL [range, 0.3–13.5 ng/mL] vs. 1.2 ± 1.9 ng/mL [range, 0.3–6.5 ng/mL], P = 0.034). 68Ga-NeoB PET/MRI was negative in 9 (33.3%) of 27 participants, with 3 (33.3%) of these 9 being false-negative (Table 3).
SUV of Lesions and Organs for 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI and Their Significance
Diagnostic Performance of 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI in Detection of Recurrent PC on Per-Patient Basis
68Ga-PSMA-R2 PET/MRI
68Ga-PSMA-R2 PET/MRI showed 20 lesions in 15 (55.6%) of 27 patients: 1 (5%) of these 20 lesions was local recurrence in the prostate, 6 (30%) were pelvic nodal, 2 (10%) were extrapelvic nodal, 10 (50%) were bone, and 1 (5%) was a lung metastasis. All 68Ga-PSMA-R2 PET–positive patients were confirmed true-positive using the reference standard (Supplemental Table 2). Mean lesion SUVmax was 4.4 ± 1.5 (range, 2.6–8.8), with a mean tumor-to-background ratio of 7.4 ± 3.1 (range, 3.4–15.1). All SUVs are summarized in Table 2 and Supplemental Table 3. The PSA levels for patients with positive 68Ga-PSMA-R2 findings were not different from those of patients with negative 68Ga-PSMA-R2 findings (3.9 ± 4.1 ng/mL [range, 0.3–13.5 ng/mL] vs. 1.9 ± 2.8 ng/mL [range, 0.3–9.3 ng/mL], P = 0.156). 68Ga-PSMA-R2 PET/MRI was negative in 12 (44.4%) patients but false-negative in 6 (50%) of these 12 (Table 3).
68Ga-NeoB PET/MRI Versus 68Ga-PSMA-R2 PET/MRI
The detection rate for 68Ga-NeoB PET/MRI was not significantly higher than that of 68Ga-PSMA-R2 on either a per-patient basis (66.7% vs. 55.6%, P = 0.083) or a per-lesion basis (31 vs. 20 lesions, P = 0.141). There were 17 true-positive lesions that were detected by both 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI (Fig. 1). There were also 17 lesions that were incongruent between the 2 radiopharmaceuticals: 14 (82%) of 17 were positive on 68Ga-NeoB and negative on 68Ga-PSMA-R2 PET/MRI (Fig. 2), correlating to 1 seminal vesicle lesion, 4 pelvic and 2 extrapelvic nodal lesions, and 7 bone metastases, which were true-positive according to the standard reference; 3 (18%) of 17 lesions were positive on 68Ga-PSMA-R2 but negative on 68Ga-NeoB PET/MRI, correlating to 1 extrapelvic lymph node and 2 bone metastases (Fig. 3). These lesions were also true-positive according to the reference standard but did not change overall detection rates on a per-patient level. Three participants were negative on both scans, as verified by the reference standard, and were subsequently under active surveillance.
Venn diagram of lesion detected on 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI, by site. All identified lesions were true-positive.
71-y-old man with BCR PC after radical prostatectomy (from left to right: axial PET, axial PET/MRI, axial MRI, and maximum-intensity projection). PSA at time of PET/MRI was 3.63 ng/mL. 68Ga-NeoB PET/MRI (top row) shows intense uptake in left seminal vesicle and pelvic lymph node (arrows), whereas 68Ga-PSMA-R2 PET/MRI (bottom row) was negative. Lesions were confirmed on follow-up 18F-DCFPyL PET/CT and by PSA decrease as treatment response after initiation of androgen deprivation therapy. 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI were performed within 2 d.
76-y-old man with BCR PC after radical prostatectomy (from left to right: axial PET, axial PET/MRI, axial MRI, and maximum-intensity projection). PSA at time of PET/MRI was 8.26 ng/mL. 68Ga-PSMA-R2 PET/MRI (top row) shows uptake in right hilar lymph node (arrows), whereas 68Ga-NeoB (bottom row) was negative. Additional lung metastasis (not shown) was seen with both radiopharmaceuticals. Patient was treated with androgen deprivation therapy, with consecutive PSA decrease. PET/MRI scans were performed within 14 d.
68Ga-NeoB PET/MRI had a higher sensitivity (85.7% vs. 71.4%), accuracy (88.9% vs. 77.8%), and negative predictive value (66.7% vs. 50.0%) than did 68Ga-PSMA-R2, whereas specificity and positive predictive value were equally high (100.0% for both) (Table 3). In 6 patients, 68Ga-NeoB detected 14 lesions that were false-negative on 68Ga-PSMA-R2 PET/MRI (Figs. 4 and 5).
67-y-old man with BCR PC after radiation therapy (from left to right: sagittal PET, sagittal PET/MRI, sagittal MRI, and maximum-intensity projection). PSA at time of PET/MRI was 9.3 ng/mL. 68Ga-NeoB PET/MRI (top row) shows multiple foci in skeleton: T10, T12, L1, sacrum, and right eighth rib, as well as additional left paratracheal lymph node metastasis (blue arrows). High uptake is seen in gallbladder (physiologic as means of hepatobiliary excretion) and in distal esophagus (green arrows). 68Ga-PSMA-R2 PET/MRI (bottom row) was negative. Lesions were confirmed on subsequent 18F-DCFPyL PET/CT and by PSA decrease after combined treatment of radiation therapy and androgen deprivation therapy. PET/MRI scans were performed within 7 d.
55-y-old man with BCR PC after radical prostatectomy (from left to right: axial PET, sagittal PET/MRI, axial MRI, and maximum-intensity projection). PSA at time of PET/MRI was 0.3 ng/mL. 68Ga-NeoB PET/MRI (top row) shows intense uptake in sixth cervical vertebra (arrows), whereas 68Ga-PSMA-R2 (bottom row) was negative despite 40 d between scans (no treatment initiated). Lesion was confirmed on subsequent 18F-DCFPyL PET/CT and by PSA decrease after androgen deprivation therapy.
The lesional SUVmax and tumor-to-background ratio of 68Ga-NeoB were significantly higher than those of 68Ga-PSMA-R2 (P = 0.019 and 0.013, respectively). All background tissues, gluteal muscle and bone, showed similar tracer uptake, whereas blood pool activity and liver uptake were significantly lower for 68Ga-PSMA-R2.
PSA
Differences in PSA values between positive 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI findings, as well as between negative 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI findings, were not significant (P = 0.156 and P = 0.550, respectively).
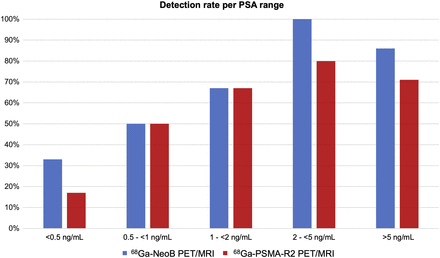
The detection rates of 68Ga-NeoB PET/MRI per PSA range (<0.5 ng/mL, 0.5 to <1.0 ng/mL, 1.0 to <2.0 ng/mL, 2.0 to <5.0 ng/mL, and ≥5.0 ng/mL) were higher than those of 68Ga-PSMA-R2 (33.3%, 50.0%, 66.7%, 100.0%, and 85.7% vs. 16.7%, 50.0%, 66.7%, 80.0%, and 71.4%, respectively) (Fig. 6) as well as per PSA doubling time (<3 mo, 3 to <6 mo, 6 to <9 mo, 9 to <12 mo, and ≥12 mo: 100.0%, 66.7%, 50.0%, 33.3%, and 33.3% vs. 85.7%, 50.0%, 50.0%, 0.0%, and 33.3%, respectively) (Supplemental Table 4).
Detection rates of 68Ga-NeoB and 68Ga-PSMA-R2 PET/MRI per PSA range. 68Ga-NeoB performed better overall, particularly at low PSA level.
DISCUSSION
Here, we assessed the feasibility, safety, and diagnostic performance of 68Ga-NeoB and 68Ga-PSMA-R2 at the time of BCR PC when findings on conventional imaging were negative or equivocal. This specific cohort is clinically challenging to manage because treatment can be given only blindly as radiation therapy to the pelvis or systemic androgen deprivation therapy. Both 68Ga-NeoB and 68Ga-PSMA-R2 were feasible and safe for diagnostic imaging. 68Ga-NeoB PET/MRI showed higher detection rates on a per-patient and per-lesion basis than did 68Ga-PSMA-R2, along with a higher sensitivity, accuracy, and negative predictive value. Specificity and positive predictive value were equally high.
Both radiopharmaceuticals, 68Ga-PSMA-R2 and 68Ga-NeoB, were developed in an effort to advance compounds that are suitable for theranostics. Preliminary results for 68Ga-PSMA-R2 showed a low absorbed radiation dose in the salivary glands, indicating its potential for therapeutic use (10). Our results corroborate low uptake of 68Ga-PSMA-R2 in the parotid gland. However, lower tumor uptake was also noted as expressed by the low tumor-to-background ratio. In comparison to 68Ga-PSMA-11 and 18F-DCFPyL, lower uptake in the salivary glands, liver, and tumor lesions was seen with 68Ga-PSMA-R2 (22). This suggests that 68Ga-PSMA-R2 might not be an ideal theranostic compound. The overall diminished uptake may have contributed to lower detection rates and sensitivity. The sensitivity of 71% for 68Ga-PSMA-R2 lies on the lower end of the published pooled sensitivity of 70%–95.5% for 68Ga-PSMA-11 and 18F-DCFPyL but is comparable to the detection rate of 61% from the PROfind trial for 68Ga-PSMA-R2 PET/CT in 18 patients with BCR PC. Specificity compares with the published pooled specificity of 70%–100% (23–27). However, in the selected cohort of BCR PC patients with prior negative or equivocal conventional imaging results, a lower detection rate of 57.6% was reported for 18F-DCFPyL (23).
68Ga-NeoB has an improved, higher affinity for the GRPR, on which it acts as an antagonist. The highest physiologic uptake was seen in the pancreas, followed by the liver, as is consistent with data from a phase I/IIa study evaluating 68Ga-NeoBOMB1 in gastrointestinal stromal tumors (28). Compared with 68Ga-RM2, the currently most investigated GRPR antagonist, tumor uptake of 68Ga-NeoB was comparable, whereas physiologic uptake was lower in the pancreas and higher in the liver (29). The sensitivity of 86% for 68Ga-NeoB is higher than what has been reported for 68Ga-RM2 PET/MRI at the time of biochemical recurrence, with detection rates ranging from 70% to 75% in larger cohorts (14,17,30) and 63% for 68Ga-RM2 PET/CT in a small cohort of 16 patients with BCR PC and noncontributory 18F-fluoroethylcholine PET/CT results (12). Taken together, these findings indicate that 68Ga-NeoB seems to be a suitable theranostic compound.
The detection rate of 68Ga-NeoB, stratified by the respective PSA ranges, was higher at low PSA levels. The detection rates increased with increasing PSA for both GRPR- and PSMA-targeting radiopharmaceuticals. In line with the overall lower detection rate of 68Ga-PSMA-R2, the rates of localizing disease by PSA range and PSA doubling time was also lower than seen in previously published data for 18F-DCFPyL (31) and 68Ga-PSMA-11 (14). Despite comparable sensitivity, 68Ga-NeoB showed slightly lower detection rates by PSA range than did previous findings for 68Ga-RM2 PET/MRI (14).
There are some limitations to this study: first, there were 4 participants for whom the scans could not be performed within the 2-wk frame because of restrictions caused by the coronavirus disease 2019 pandemic. However, despite the long interval between scans, no progression was seen. The imaging studies that were performed 21 and 35 d apart showed identical results, whereas the scans with a 72-d interval were both negative; the results of the PET/MRI scan that was performed 40 d after an initial positive scan were found to be false-negative. A second limitation was the lack of histopathology as a gold standard for all participants. The setback of using a composite reference is that it may lead to higher specificity calculations because there may not be a significant number of false-negative results. Nonetheless, obtaining histopathology for all potential metastatic lesions is technically unfeasible and impractical and is not ethical; treating physicians often rely on posttreatment PSA changes. A third limitation was the small patient cohort, although not uncommon for a phase II trial.
CONCLUSION
68Ga-NeoB and 68Ga-PSMA-R2 are safe radiopharmaceuticals. In the setting of BCR PC with prior noncontributory conventional imaging results, 68Ga-NeoB performed better than 68Ga-PSMA-R2 PET/MRI in localizing recurrent disease, particularly at low PSA levels. The overall lower uptake of 68Ga-PSMA-R2 in tumors and background organs might limit its use as a theranostic compound. These results need to be confirmed in larger studies.
KEY POINTS
QUESTION: What is the diagnostic performance of the GRPR-targeting 68Ga-NeoB and the PSMA-targeting 68Ga-PSMA-R2 for localization of recurrent PC?
PERTINENT FINDINGS: In this prospective phase II study, 68Ga-NeoB PET/MRI demonstrated higher sensitivity (86% vs. 71%), accuracy (89% vs. 78%), and negative predictive value (67% vs. 50%) than 68Ga-PSMA-R2 PET/MRI. Specificity and positive predictive value were equally high for both radiopharmaceuticals (100%). 68Ga-NeoB PET/MRI detected 14 lesions in 6 patients whose imaging results were false-negative with 68Ga-PSMA-R2.
IMPLICATIONS FOR PATIENT CARE: Both 68Ga-NeoB and 68Ga-PSMA-R2 are safe radiopharmaceuticals that have shown high and accurate diagnostic performance in evaluating biochemical recurrence of PC. Interrogating 2 targets, PSMA and GRPR, at a stage when the disease is characterized by high tumor heterogeneity may ultimately allow for personalized medicine.
DISCLOSURE
The study was supported by Novartis. Andrei Iagaru reports institutional research support and consulting fees from Novartis. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Apr. 25, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 5, 2023.
- Revision received March 15, 2024.