Abstract
185
Objectives: Ra-223, a targeted alpha therapy, has shown a survival benefit and a favorable safety profile over a 3-year follow-up period in pts with mCRPC enrolled in the phase 3 ALSYMPCA trial. REASSURE (NCT02141438) is a global, prospective, single-arm, observational study investigating the safety of Ra-223 in routine clinical practice in pts with mCRPC and is planned to cover a 7-year follow-up period.
Methods: The primary outcome measures for REASSURE were incidence of second primary malignancy (SPM) and short- and long-term safety in pts who received ≥1 Ra-223 dose; secondary outcomes included pain severity and pain assessment over time using the Brief Pain Inventory-Short Form (BPI-SF) questionnaire. A clinically meaningful pain response was determined by the worst pain item on the BPI-SF (defined as an improvement [decrease] of ≥2 points from baseline). Concomitant radiotherapy, treatments for hematologic adverse events (AEs) up to 6-months after last administration of Ra-223, and subsequent chemotherapy use were also recorded. Herein, results from the second prespecified interim analysis are presented.
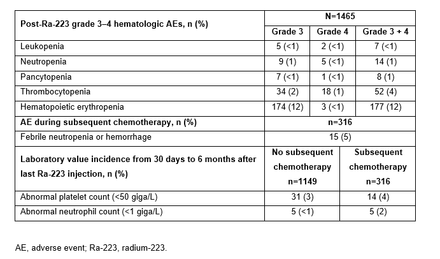
Results: A total of 1465 pts were included in the safety analysis; median follow up was 11.5 months. The median number of Ra-223 doses received by pts was 6. Overall, 14/1465 pts (1%) developed ≥1 SPM. 160/1465 pts (11%) received radiotherapy concomitant with Ra-223; of these, 125/160 pts (78%) received conventional external beam radiotherapy. Other radiotherapy modalities included brachytherapy (n=14 [8.8%]), gamma knife (n=8 [5.0%]), missing (n=19 [11.9%]), and other (n=2 [1.3%]). 316/1465 pts (22%) received chemotherapy subsequent to Ra-223 and 1149/1465 (78%) did not; 128/316 pts (41%) and 269/1149 pts (23%) received ≥1 treatment for hematologic AEs, respectively. Furthermore, 214/1465 pts (15%) experienced a post-Ra-223 grade 3-4 hematologic AE (Table); out of these, 26/214 had grade 4. Rates of abnormal platelet and neutrophil counts were similar between pts who received subsequent chemotherapy and those who did not (Table). At treatment 6, the mean change from baseline in BPI pain severity score was −0.36 in 653 evaluable pts. 222/657 pts (34%) had a clinically meaningful pain response in the BPI-SF worst pain item, at treatment 6.
Conclusions: Up to the second prespecified interim analysis of the REASSURE study, there was a low overall incidence of SPM, including in pts receiving concomitant radiotherapy or subsequent chemotherapy, and low overall rates of post-Ra-223 grade 3-4 hematologic AEs. Pts were able to achieve an improvement in pain after treatment with Ra-223. This study confirms that Ra-223 can be utilized in routine clinical practice as part of standard therapy for mCRPC, with meaningful impact on bone pain. No new safety concerns were observed. AE, adverse event; Ra-223, radium-223.