Abstract
111
Objectives: Cardiopulmonary Resuscitation (CPR) following cardiac arrest (CA) can lead to neurological deficits ranging from minor cognitive impairments to persistent vegetative state and brain death. Although the resulting brain injury has been well documented in clinical cases and preclinical models, its pathophysiology remains poorly understood. Previous studies have focused primarily on post-arrest changes in in cerebral blood flow or neuroanatomy and few have investigated post arrest changes in brain metabolism. Large fluctuations in cerebral glucose metabolism (CGM) have been reported during and immediately following cerebral ischemia but how these patterns are affected in the context of post-cardiac arrest resuscitation are unknown. In this study we utilized 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG-PET) to study in vivo cerebral glucose metabolism in the post-CA setting.
Methods: Anesthetized and ventilated adult C57BL/6 mice were undergone 8-minute KCl-induced CA followed by 90 second cardiopulmonary resuscitation and administration of epinephrine. Surviving mice were intraperitoneally injected with 18F-FDG (~250 uCi/250 ul) 72-hours after the CA and imaged thirty minutes later by high resolution CT imaging on a Molecube’s preclinical imaging system. CT imaging was used as anatomic templates. Brain FDG uptake was determined by Invicro VivoQuant software on fused PET/CT images with the 3D brain atlas. Upon completion of PET imaging, remaining FDG radioactivity in the brain, heart, and liver was determined by isolating organs and measuring 18F activity using a gamma counter. The FDG uptake was expressed as percent of injected dose per gram of tissue (%ID/g).
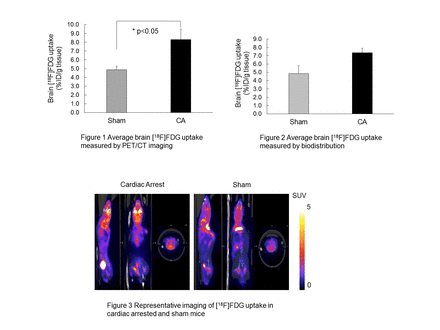
Results: A global increase in FDG uptake in the brains of CA mice was observed compared to the sham group. The PET imaging shows that on average, the normalized uptake of FDG (%ID/g) for CA animals was 7.9%, which is statistically higher than the sham animals (4.89%) (p<0.05). In addition, this increased uptake was consistent throughout 60-min imaging period and across all the brain regions. Biodistribution analyses of various key organs yielded similar observations for brain FDG uptake (7.58% for CA mice vs 5.80% for sham animals).
Conclusions: This study demonstrates for the first time, successful application of using PET/CT imaging to measure changes in brain metabolism, utilizing brain FDG uptake, in a murine model of CA. Our results suggest increased FDG uptake in the brain following cardiac arrest indicating altered patterns of cellular metabolism. Further studies are necessary to determine the implications of this altered metabolic pattern.