Abstract
Linker instability and impaired tumor targeting can affect the tolerability and efficacy of antibody–drug conjugates (ADCs). To improve these ADC characteristics, we recently described the use of a metal–organic linker, [ethylenediamineplatinum(II)]2+, herein called Lx. Initial therapy studies in xenograft-bearing mice revealed that trastuzumab-Lx-auristatin F (AF) outperformed its maleimide benchmark trastuzumab-mal-AF and the Food and Drug Administration–approved ado-trastuzumab emtansine, both containing conventional linkers. In this study, we aimed to characterize Lx-based ADCs for in vivo stability and tumor targeting using 195mPt and 89Zr. Methods: The γ-emitter 195mPt was used to produce the radiolabeled Lx [195mPt]Lx. 89Zr-Desferrioxamine (89Zr-DFO) was conjugated to trastuzumab either via [195mPt]Lx (to histidine residues) or conventionally (to lysine residues) in order to monitor the biodistribution of antibody, payload, and linker separately. Linker stability was determined by evaluating the following ADCs for biodistribution in NCI-N87 xenograft–bearing nude mice 72 h after injection: trastuzumab-[195mPt]Lx-DFO-89Zr, trastuzumab-[195mPt]Lx-AF, and 89Zr-DFO-(Lys)trastuzumab (control), all having drug-to-antibody ratios (DARs) of 2.2–2.5. To assess the influence of DAR on biodistribution, 89Zr-DFO-(Lys)trastuzumab-Lx-AF with an AF-to-antibody ratio of 0, 2.6, or 5.2 was evaluated 96 h after injection. Results: Similar biodistributions were observed for trastuzumab-[195mPt]Lx-DFO-89Zr, trastuzumab-[195mPt]Lx-AF, and 89Zr-DFO-(Lys)trastuzumab irrespective of the isotope used for biodistribution assessment. The fact that Lx follows the antibody biodistribution indicates that the payload-Lx bond is stable in vivo. Uptake of the 3 conjugates, as percentage injected dose (%ID) per gram of tissue, was about 30 %ID/g in tumor tissue but less than 10 %ID/g in most healthy tissues. Trastuzumab-[195mPt]Lx-AF (DAR 2.2) showed a tendency toward faster blood clearance and an elevated liver uptake, which increased significantly to 28.1 ± 4.2 %ID/g at a higher DAR of 5.2, as revealed from the biodistribution and PET imaging studies. Conclusion: As shown by 195mPt/89Zr labeling, ADCs containing the Lx linker are stable in vivo. In the case of trastuzumab-Lx-AF (DARs 2.2 and 2.6), an unimpaired biodistribution was demonstrated.
Four antibody–drug conjugates (ADCs) have been approved by the Food and Drug Administration (Adcetris [brentuximab vedotin; Seattle Genetics] (1), Kadcyla [ado-trastuzumab emtansine; Genentech] (2), Mylotarg [gemtuzumab ozogamicin; Pfizer], and Besponsa [inotuzumab ozogamicin; Pfizer] (3)), and more than 70 are currently under clinical evaluation (4). Nevertheless, approvals are stagnating, and several ADCs have failed to be approved because of safety issues or insufficient efficacy (5). Therefore, a deeper insight into the in vivo performance of ADCs is clearly desirable.
The linker system used for coupling of the drug to the monoclonal antibody is of key importance for efficacy but also for the tolerability and safety of an ADC (6–9). First, the drug can be released from the monoclonal antibody into the circulation, resulting in unwanted sequestration of the drug in healthy tissues (10,11). Second, the antibody itself can be affected by drug conjugation, especially at a high drug-to-antibody ratio (DAR), resulting in impaired tumor binding or faster blood clearance of the ADC, with uptake in catabolic organs such as liver and spleen (12,13). Third, after cellular uptake of an ADC and subsequent catabolism, the drug can become detached from the antibody and eventually be released from the cell (5,14–16). It can subsequently kill neighboring cancer cells (bystander effect), but it can also result in increased toxicity by systemic exposure.
Most linker systems currently used in clinical studies are based on conventional active ester or maleimide chemistry for drug conjugation to lysine or cysteine residues of the monoclonal antibody, respectively. It has been recognized that these linkers provide suboptimal ADCs; therefore, extensive research on new conjugation technologies has been initiated during the last few years (5,17–19). As a pioneering approach in the development of ADCs, we recently described the use of the cationic metal–organic linker [ethylenediamineplatinum(II)]2+, herein called Lx (LinXis) (20,21). In a first step, Lx can be coordinated to payloads bearing nonconventional functionalities, such as an N-heterocyclic ligand, to provide storable products that we term semifinal. In a second step, an Lx-drug semifinal product is conjugated efficiently to histidine residues of unmodified monoclonal antibodies. On the basis of these characteristics, the Lx linker technology can pave the way to a plug-and-play ADC development platform in which antibodies and payloads can easily be varied. The potential of the Lx linker technology was recently demonstrated in the preparation of auristatin F conjugated trastuzumab (trastuzumab-Lx-AF) (21). A single dose of trastuzumab-Lx-AF outperformed its maleimide benchmark trastuzumab-mal-AF and the Food and Drug Administration–approved ado-trastuzumab emtansine in a xenograft mouse model of gastric cancer (NCI-N87) and of ado-trastuzumab emtansine–resistant breast cancer (JIMT-1). Nevertheless, an in-depth analysis of the in vivo performance of Lx-based ADCs is needed to disclose the distinguished properties of Lx in more detail.
In general, for the assessment of in vivo stability and tumor targeting of an ADC, tissue samples are taken for analytic evaluation of ADC integrity and uptake, for example, by enzyme-linked immunosorbent type assays or liquid chromatography–mass spectrometry/mass spectrometry (22–25). However, such procedures are invasive, time consuming, difficult to apply for longitudinal whole-body analysis including tumor uptake analysis, and inaccurate with respect to quantification.
For the in vivo characterization of Lx-based ADCs, we therefore present an alternative approach that exploits an integrated strategy of dual radiolabeling and immuno-PET imaging using the radionuclides 89Zr and 195mPt. In previous studies, we have demonstrated how 89Zr-immuno-PET can be used for the in vivo evaluation of biologicals, including ADCs (26–28). For this purpose, we have developed generic good-manufacturing-practice–compliant labeling methods using the bifunctional chelator desferrioxamine (DFO) (Desferal; Novartis) for coupling of 89Zr to antibodies (29,30). Here, we show for the first time how 195mPt, produced in the high-flux reactor in Petten (Supplemental Table 1 shows the batch characteristics of the delivered 195mPt-complex; supplemental materials are available at http://jnm.snmjournals.org), can be used to obtain the radioactive [195mPt]Lx linker (31). 195mPt emits low-energy γ-radiation and has a half-life of 4.02 d, which matches the biologic half-life of antibodies. The combination of 195mPt and 89Zr allows sensitive and direct detection of the Lx linker next to the antibody and the drug payload (for this purpose, 89Zr-DFO was used as a model) at low concentrations in tissue samples. The radiolabels 89Zr and 195mPt were exploited to demonstrate the in vivo stability of Lx-based ADCs and the in vivo uptake and retention of Lx-based ADCs in tumors and healthy tissues in relation to DAR. To characterize Lx as an ADC linker in vivo, 89Zr-DFO was conjugated to trastuzumab either via [195mPt]Lx (to histidine residues) or conventionally (to lysine residues). The following constructs were evaluated in comparative biodistribution and imaging studies: trastuzumab-[195mPt]Lx-DFO-89Zr, trastuzumab-[195mPt]Lx-AF, 89Zr-DFO-(Lys)trastuzumab-Lx-AF, and 89Zr-DFO-(Lys)trastuzumab (control).
MATERIALS AND METHODS
Cell Lines and General Materials and Procedures
This information is available from the authors on request.
Synthesis of [195mPt]Lx-Based Conjugates
Details on the chemical structures, synthesis, and analysis of constructs 1–3 (Fig. 1A) used for in vivo evaluation are available from the authors on request.
Structural representation of constructs 1–6.
Synthesis of 89Zr-DFO-(Lys)Trastuzumab-Lx-AF with Different AF-to-Antibody Ratios
Details on the synthesis of 89Zr-DFO-(Lys)trastuzumab-Lx-AF with AF-to-antibody ratios of 2.6 (5) and 5.2 (6) (Fig. 1B) are available from the authors on request. As a control for biodistribution and imaging studies, 89Zr-DFO-(Lys)trastuzumab (4) (Fig. 1B), synthesized as described previously (29), was included.
Analytic Procedures
Procedures to assess the conjugation efficiency and the DAR and to analyze the quality of the radiolabeled conjugates are available from the authors on request.
Dual-Isotope 195mPt and 89Zr Counting
To determine the optimal energy windows for simultaneous 195mPt and 89Zr counting, the energy spectrums of both isotopes were assessed using an automatic γ-counter (Hidex). The energy windows for counting were set at 30–210 keV and 400–1,100 keV for 195mPt and 89Zr, respectively. To determine the activity of 195mPt in the dual-isotope experiments, the activity measured in the 30–210 keV energy window needed to be corrected for the activity related to 89Zr in this energy window using Equation 1: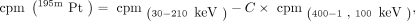 Eq. 1in which
Eq. 1in which Since 195mPt does not emit photons in the 89Zr energy window, the activity measured in this window can be directly related to 89Zr using Equation 2:
Since 195mPt does not emit photons in the 89Zr energy window, the activity measured in this window can be directly related to 89Zr using Equation 2: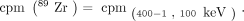 Eq. 2
Eq. 2
Biodistribution of [195mPt]Lx-Based Conjugates
The biodistribution of constructs 1–3 was evaluated in nude mice bearing the human epidermal growth factor receptor 2–positive gastric cancer xenograft line NCI-N87. All animal experiments were performed according to the National Institutes of Health Principles of Laboratory Animal Care and Dutch national law (“Wet op de dierproeven,” Staatsblad 1985, 336). Nineteen days before administration of the constructs, 19 female mice (HSD:Athymic Nude-Foxn1nu, 21–31 g [Harlan]; 8–10 wk old at the time of the experiments) were injected subcutaneously with 2.5 × 106 NCI-N87 cells in both flanks. Mice bearing NCI-N87 xenografts (tumor volume 100–250 mm3) were anesthetized by inhalation of 2% isoflurane and injected intravenously via the retroorbital plexus (32) with the radiolabeled constructs (∼4 mg/kg for ADCs 1–3) in a 100 μL injection volume. The injected 195mPt and 89Zr radioactivity doses were chosen in such a way that an accurate correction of the 195mPt signal in the γ-counter for the contribution of 89Zr counts was possible. These requirements were crucial during synthesis and radiolabeling and resulted in the samples for injection as presented in Table 1.
Characteristics of Radiolabeled Constructs 1–6 Evaluated in Tumor-Bearing Mice
Blood was collected via the tail 2, 24, and 48 h after injection of the tracer. At the end of the experiment, 72 h after injection, the mice were anesthetized, bled, sacrificed, and dissected. After blood, tumor, and healthy tissues had been weighed, the amount of radioactivity in each sample was measured in a γ-counter for each radioisotope present. Radioisotope uptake was calculated as the percentage of the injected dose per gram of tissue (%ID).
Biodistribution of 89Zr-Labeled Lx-Based ADCs with Different AF-to-Antibody Ratios
The biodistribution of 89Zr-DFO-(Lys)trastuzumab-Lx-AF constructs 5 and 6 with different AF-to-antibody ratios was evaluated in NCI-N87 tumor–bearing mice and compared with the biodistribution of 89Zr-DFO-(Lys)trastuzumab (4). All conjugates (Fig. 1B) were prepared with a relatively low 89Zr-DFO–to–antibody ratio of approximately 1 (Table 1) to minimize a potential contribution of 89Zr-DFO to pharmacokinetic effects.
After administration of the conjugates, PET imaging was performed with a dedicated small-animal NanoPET/CT scanner (Mediso Ltd.). A mouse of each group was anesthetized by inhalation of 2% isoflurane and scanned 96 h after injection for 1 h. A CT scan was acquired before the PET scan and used for attenuation and scatter correction. Reconstruction was performed with a fully 3-dimensional algorithm (Tera-Tomo; Mediso Ltd.) with 4 iterations and 6 subsets, resulting in an isotropic 0.4-mm voxel dimension.
Statistical Analysis
All animal experiments were statistically analyzed using the Welch t test for independent samples. Two-sided significance levels were calculated, and a P value of less than 0.05 was considered statistically significant.
RESULTS
Rationale for Preparation of Radiolabeled Constructs
Three constructs (Fig. 1A) were prepared to allow characterization of the Lx linker in biodistribution studies. For this purpose, either the diagnostic payload 89Zr-DFO or the therapeutic payload AF was coupled to trastuzumab via [195mPt]Lx (Table 1). By assessment of 195mPt and 89Zr counts, crucial information on the Lx in vivo performance was expected to be obtained from the following constructs: trastuzumab-[195mPt]Lx-DFO-89Zr (1), 89Zr-DFO-(Lys)trastuzumab (2), and trastuzumab-[195mPt]Lx-AF (3).
The evaluation of conjugate 1 gives information on the stability of Lx-based ADCs in blood and at the tumor site and on sequestration of putative Lx-containing metabolites along the body, as is important for the tolerability of Lx-based ADCs. Subsequently, the comparison of conjugates 1 and 2 provides information about stability of the metal–organic linker Lx compared with a classic covalent organic linker. Further, the comparison of conjugates 1 and 3 allows determination of the biodistribution and tumor uptake of Lx-based ADCs related to the type of payload: the diagnostic moiety 89Zr-DFO or the therapeutic drug AF. Finally, 89Zr-labeled AF-bearing constructs 5 and 6 along with an AF-free reference construct 4 (Fig. 1B), are used to evaluate the effect of AF-to-antibody ratio on the biodistribution.
Biodistribution of [195mPt]Lx–Based Conjugates
Biodistribution of conjugates 1–3 was assessed in nu/nu mice bearing NCI-N87 xenografts at 72 h after injection. All tissue and blood values are presented in Supplemental Tables 2–7.
Biodistribution of Trastuzumab-[195mPt]Lx-DFO-89Zr (1)
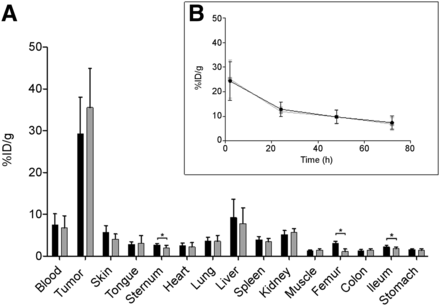
Biodistribution analysis of trastuzumab-[195mPt]Lx-DFO-89Zr (1) revealed similar levels of 195mPt and 89Zr in blood, tumors, and most of the healthy tissues (Fig. 2A). In sternum and femur, a higher uptake of 89Zr than of 195mPt was found, as can be explained by minor instability of the 89Zr-DFO complex and the osteophilic properties of unbound 89Zr (33). Similar blood levels were confirmed by analysis of blood kinetics (Fig. 2B). These results confirm the in vivo stability of the coordinative bond between the Lx linker and the payload.
Biodistribution 72 h after injection (A) and blood kinetics (B) of 4 mg/kg dose of trastuzumab-[195mPt]Lx-DFO-89Zr (1, DAR 2.4; n = 7) in NCI-N87 xenograft–bearing mice. Black bars and line = 89Zr counting; gray bars and line = 195mPt counting.
Biodistribution of Trastuzumab-[195mPt]Lx-DFO-89Zr (1) Versus 89Zr-DFO-(Lys)trastuzumab (2)
The biodistribution of trastuzumab-[195mPt]Lx-DFO-89Zr (1) was compared with the biodistribution of 89Zr-DFO-(Lys)trastuzumab (2) on the basis of 89Zr counting (Fig. 3A). Both constructs demonstrated a similar biodistribution irrespective of whether 89Zr-DFO was conjugated via [195mPt]Lx to histidine residues or via classic conjugation to the lysine residues of the antibody. Nevertheless, a faster blood clearance of the Lx-based construct was observed, accompanied by a slightly increased liver uptake. The tendency for a faster blood clearance was confirmed by pharmacokinetic analyses (Fig. 3B). The combined results of Figures 2 and 3 show that the biodistribution of the [195mPt]Lx linker follows the biodistribution of the antibody, indicating that Lx-based ADCs are stable in vivo. Nevertheless, conjugation to histidine residues instead of conjugation to lysine residues slightly alters the pharmacokinetics.
Biodistribution 72 h after injection (A) and blood kinetics (B) of 4 mg/kg dose of trastuzumab-[195mPt]Lx-DFO-89Zr (1, DAR 2.4; n = 7) and 89Zr-DFO-(Lys)trastuzumab (2, DAR 2.5; n = 6) in NCI-N87 xenograft–bearing mice, both assessed by 89Zr counting. Black bars and line = trastuzumab-[195mPt]Lx-DFO-89Zr (1); gray bars and line = 89Zr-DFO-(Lys)trastuzumab (2).
Biodistribution of Trastuzumab-[195mPt]Lx-DFO-89Zr (1) Versus Trastuzumab-[195mPt]Lx-AF (3)
The biodistribution of trastuzumab-[195mPt]Lx-DFO-89Zr (1) and trastuzumab-[195mPt]Lx-AF (3) was compared on the basis of 195mPt counting (Fig. 4A). Despite the different payloads, 89Zr-DFO or AF, both conjugates showed similar blood levels, tumor uptake, and uptake in most of the dissected tissues. Only in the liver did trastuzumab-[195mPt]Lx-AF (3) show a higher uptake than trastuzumab-[195mPt]Lx-DFO-89Zr (1): 17.0 ± 2.2 %ID/g versus 7.8 ± 3.8 %ID/g, respectively. Similar blood levels were confirmed by pharmacokinetic analyses (Fig. 4B). These results indicate that Lx-based ADCs are stable in vivo irrespective of the nature of the payload but that the nature of the payload might affect the biodistribution.
Biodistribution 72 h after injection (A) and blood kinetics (B) of 4 mg/kg dose of trastuzumab-[195mPt]Lx-DFO-89Zr (1, DAR 2.4; n = 7) and trastuzumab-[195mPt]Lx-AF (3, DAR 2.2; n = 6) in NCI-N87 xenograft–bearing mice, both assessed by 195mPt counting. Black bars and line = trastuzumab-[195mPt]Lx-DFO-89Zr (1); gray bars and line = trastuzumab-[195mPt]Lx-AF (3).
Biodistribution of 89Zr-DFO-(Lys)trastuzumab-Lx-AF with Different AF-to-Antibody Ratios
We found that, compared with the ADC containing 89Zr-DFO as the payload, coupling of AF to histidine residues of trastuzumab via Lx with a DAR of 2.2 caused slightly faster clearance of the ADC from the blood, accompanied by a higher liver uptake (Fig. 4). This finding indicates that a DAR of around 2.2 might be the maximum for optimal tumor targeting. To explore this assumption, 89Zr-DFO-(Lys)trastuzumab-Lx-AF conjugates with an AF-to-antibody ratio of 0 (4), 2.6 (5), and 5.2 (6) were evaluated in biodistribution (Fig. 5A) and PET imaging studies (Fig. 5C) in mice bearing NCI-N87 xenografts 96 h after injection. In addition, blood kinetics (Fig. 5B) were assessed up to 96 h after injection.
Biodistribution 96 h after injection (A), blood kinetics (B), and PET images 96 h after injection (C) of 89Zr-DFO-trastuzumab-Lx-AF, with AF-to-antibody ratios of 0 (4), 2.6 (5), and 5.2 (6), to NCI-N87 xenograft–bearing mice. Black bars, black line, and left PET image = AF-to-antibody ratio of 0 (4); red bars, red line, and middle PET image = AF-to-antibody ratio of 2.6 (5); blue bars, blue line, and right PET image = AF-to-antibody ratio of 5.2 (6).
Conjugate 6, with the highest DAR (5.2), demonstrated impaired tumor targeting, faster blood clearance, and increased liver uptake (Fig. 5A) than conjugate 4 (DAR 0), as was confirmed by the analysis of blood kinetics (Fig. 5B) and by PET imaging (Fig. 5C). Also, conjugate 5 (DAR 2.6) showed such a tendency, although no statistical differences were observed compared with conjugate 4 (Fig. 5). All tissue and blood values are presented in Supplemental Tables 8 and 9.
DISCUSSION
Recently, we introduced Lx as a promising metal–organic ADC linker (20,21). In a first step, Lx is coordinated to diagnostic or therapeutic payloads to provide storable Pt(II) complexes, or semifinal products. In a second step, such an Lx-payload complex is conjugated to an antibody, followed by a posttreatment step with thiourea to remove all weakly bound platinum complexes. Extensive in vitro analysis using 89Zr-DFO and AF as the payloads revealed that about 85% of an Lx-payload species binds to the Fc region, presumably to histidines. The formed ADCs were found to be stable in phosphate-buffered saline and human serum without loss of the antibody binding affinity, and preliminary in vivo studies with trastuzumab-Lx-DFO-89Zr indicated pharmacokinetics and tumor-targeting properties similar to the parental trastuzumab. Finally, trastuzumab-Lx-AF appeared to be remarkably effective in therapy studies with NCI-N87 and JIMT-1 xenograft–bearing nude mice, a reason we aimed for a further disclosure of the distinguished properties of Lx in vivo.
In the current study, we explored a unique possibility arising from the availability of radioactive platinum, 195mPt, making it feasible to evaluate the in vivo performance of Lx in an accurate and facile way by radiolabeling each of the ADC components: the antibody (by 89Zr-DFO coupling to lysine residues of trastuzumab), the linker (using [195mPt]Lx), and the payload (using 89Zr-DFO as an exemplary diagnostic payload). Counting of the radioisotopes of 2 ADCs, trastuzumab-[195mPt]Lx-DFO-89Zr (1) and 89Zr-DFO-(Lys)trastuzumab (2), in biodistribution studies in NCI-N87 xenograft–bearing nude mice revealed a similar biodistribution (Figs. 2 and 3). This was also the case when a therapeutic payload (AF) was used (Fig. 4). Therefore, it can be concluded that the Lx-based ADCs are stable in serum and suitable for optimal tumor targeting. Results also revealed that Lx follows the biodistribution of an antibody and, unlike platinum-based chemotherapeutics such as cisplatin, remains an inert component of the ADC after in vivo administration during the time frame of the investigation. This information is important with respect to the tolerability and safety of Lx-based ADCs.
Comparative biodistribution studies revealed a slightly faster blood clearance of the Lx-based constructs, accompanied by an increased liver uptake (Fig. 3A). This effect became significant when the DAR of the Lx-based constructs was increased from 2.6 to 5.2 (Fig. 5). Although we and others have previously described the phenomenon of increased blood clearance in relation to DAR (12,34), it seems that Lx contributes to this phenomenon. One explanation may be an alteration of the antibody binding to the neonatal Fc receptor, which is known to be involved in recycling of IgGs and extension of their half-life in circulation (35). Histidines that are present on the Fc part of the antibody play an important role in neonatal Fc receptor binding of IgGs, and because Lx most probably conjugates to histidines, mainly in the Fc region (21), it is possible that Lx conjugation affects neonatal Fc receptor binding and the pharmacokinetics of a monoclonal antibody (36). Besides this Lx-related effect, impaired biodistribution is dependent on the number and nature of payloads, which can cause alterations in hydrophobicity, charge, or conformation.
The biodistribution studies presented here provide strong evidence that premature release of the drug or of the Lx-drug species in the blood does not occur, thus promising safety advantages for Lx-based ADCs. A second way by which toxic metabolites may give safety issues, however, is by intracellular catabolism of an ADC and release of the metabolites into the circulation. Because of the proven stability of the Lx linker, we anticipate that the drug becomes released intracellularly as a His-Lx-drug complex. After apoptosis, this metabolite may be released from the targeted cell and may enter the bloodstream (15). If such a metabolite is potent, it might cause systemic toxicity. In our previous study, we already tested the potency of Lx-AF versus AF-mal in viability assays in vitro (21). In 6 tested cell lines, the potency of Lx-AF appeared 103–104 times lower than its corresponding ADC, whereas for the maleimide-based linker and its corresponding ADC, the difference was only 10–100 times. We believe that the low toxicity of the Lx-drug complex is due to the positive charge on platinum, which impedes passage through the cell membrane. The present and previous results indicate a favorable safety profile for Lx-based ADCs. As a prelude to clinical studies, Lx-based ADCs will be rigorously evaluated in vivo in preclinical safety studies and in metabolite studies using 195mPt.
The combined use of 89Zr and 195mPt in biodistribution and imaging experiments as described here appears to be a powerful tool for the preclinical in vivo characterization of Lx-based ADCs. As demonstrated by us and others, in vivo characterization of antibodies and antibody conjugates such as ADCs by 89Zr-immuno-PET imaging can easily be translated to the clinic (26,27). By assessment of selective tumor targeting at sufficiently high uptake levels, 89Zr-immuno-PET imaging of an ADC or its antibody component might be used for personalized treatment of tumors (37–41). In contrast, at its current stage of development this is not yet possible by 195mPt imaging. The amounts of platinum used in Lx-based ADCs and the specific activity of 195mPt are too low for preclinical or clinical SPECT imaging.
CONCLUSION
Radiochemistry in combination with PET imaging is a powerful tool for ADC development and in vivo characterization. ADCs containing the Lx linker are stable in vivo, and in the case of trastuzumab-Lx-AF with DARs of 2.2 and 2.6, they showed an unimpaired biodistribution. Lx follows the biodistribution of the antibody, indicating that it remains an inert component of an ADC after its in vivo administration. This information is important with respect to the performance, tolerability, and safety of Lx-based ADCs.
DISCLOSURE
Joey Muns, Veronica Montserrat, Niels Sijbrandi, and Eugen Merkul are employed by LinXis B.V.; Hendrik-Jan Houthoff is chief executive officer of LinXis B.V. and has ownership (including patents) in LinXis B.V.; Guus van Dongen is a member of the nonprofit scientific advisory board of LinXis B.V. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Prof. Dr. Jan Reedijk (University of Leiden), an international expert in platinum chemistry, for critical reading of the manuscript.
Footnotes
Published online Mar. 1, 2018.
- © 2018 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 5, 2017.
- Accepted for publication February 3, 2018.