Abstract
615
Objectives: The use of 18Fluorodeoxyglucose (FDG) for the quantification of treatment response is more precise when variation in uptake time and blood activity is minimized between imaging time points. A target-to-background ratio (TBR) using blood as reference tissue is a quantification method intended to correct for blood pool activity, but comes with a cost of susceptibility to variation in uptake time. Some investigators propose that blood subtraction is a more robust method to remove a spatial overlap of blood activity. However, in addition to spatial overlap, apparent lesion uptake at the time of imaging is a function of the full area under the curve of FDG in the blood that is exposed to the lesion for uptake. We mathematically model this blood to target relationship followed by in vivo validation with dynamic PET images and explore the use of the liver as an alternate reference tissue.
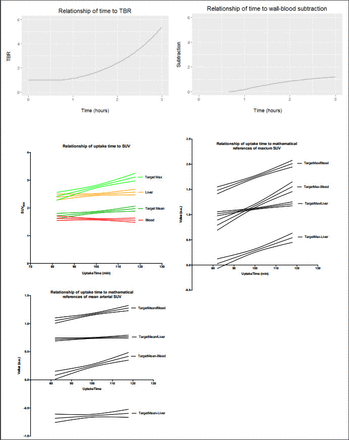
Methods: For modeling a target structure with heavy spatial overlap of blood activity, we used a 2mm thick aorta wall of low-level uptake. Patalak plotting of the time-activity curve of blood activity in the aorta lumen (population-based input function) and the target was fed to a spatial model that predicted final apparent target and blood activity. The spatial resolution of 4 full-width half-max was validated with a phantom using the same reconstruction parameters as in vivo imaging (iterative reconstruction, time-of-flight, 1.5mm slice thickness, 256 matrix). 63 dynamic (20 min) FDG PET scans were collected from 37 subjects with hyperlipidemia at 100±7 min uptake time. Image data was split into five equal 4-minute segments for a total of 315 reconstructions of a single bed position over the chest and epigastrium. Mean and maximum standardized uptake values (SUV) from the left lateral 2mm wall of the thoracic descending aorta was segmented in order to avoid esophageal or vertebral bone marrow activity. Overlap with lung was carefully avoided. Volumetric mean SUV from the right lobe of the liver and blood activity within the lumen of the right atrium was measured. Mean and maximum SUV of the artery wall was subtracted and divided by mean blood and liver activity. Plotted against uptake time, the slopes of these values were compared with respect to the percent change from 100-120 min uptake time.
Results: Based upon mathematical simulation, TBR or subtraction with blood was heavily influenced by uptake time, with an estimated 15-30% increase from 100-120 min. Respective mean (±SD) blood, liver, maximum artery wall, and mean artery wall activity for the cohort was 1.6±0.3, 2.5±0.3, 2.8±0.5, and 1.8±0.3 with a respective coefficient of variation of 16%, 14%, 17%, and 16%. In vivo plotting of measurement metrics to uptake time demonstrated similar positive relationships when blood is used with division or subtraction (10-70% increase). However, when mean artery wall activity is divided by liver activity, a favorable low response to uptake time is observed compared to all other methods of measurement (4% vs 8-101%, respectively) despite a comparably high coefficient of variation (16% and 14%, respectively) for the constituent input variables.
Conclusion: For low-level activity structures with high spatial overlap with blood such as the artery wall, division or subtraction with blood activity does not mathematically normalize for variability in uptake time or blood activity itself. Whereas, mean target artery wall activity divided by mean liver activity affords the most stable measurement value related to variability in uptake time and variability in blood activity. Subsequent modeling and outcomes-based in vivo validation will further explore the clinical translation of this information. Research Support: Supported in part by the NIH intramural research program.