Abstract
178
Objectives: PD-L1, a cell surface immune checkpoint ligand, is highly expressed in a variety of human tumors and is capable of creating an immunosuppressive environment conducive to disease progression. By overexpressing PD-L1 tumor cells can evade detection and cell death mediated by the immune system. Therapeutic anti-PD-L1 monoclonal antibodies (mAb) have been found to reverse this immunosuppressive tumor microenvironment and have shown efficacy in clinical trials in subsets of patients. Development of a PD-L1 mAb imaging agent could aid in selecting patients for these immunotherapeutics and monitoring responses. Avelumab, an anti-PD-L1 monoclonal IgG antibody (PD-L1 mAb) which recognizes both human and murine PD-L1 is one of these immunotherapeutics currently in clinical development. In these studies, avelumab was labeled with 89Zr (ZPD) and evaluated in vitro and in vivo in human PD-L1 expressing cancer cells and mouse xenografts, respectively for appropriate PD-L1 targeting and potential for clinical translation.
Methods: ZPD was synthesized using PD-L1mAb conjugated to desferrioxamine via an isothiocyanate linker (df-PD-L1mAb). In vitro binding studies (Kd, Bmax) were performed using the PD-L1+ human breast cancer cell line, MDA-MB231 (MB). ZPD biodistributions studies were performed with MB xenograft mouse models at 1, 2, 3, 5 and 7 d post ZPD coinjections without/with PD-L1 mAb dose escalation (10, 20, 40 and 400 μg) from which blood and tissue uptakes [%injected dose/g (%ID/g)] were determined. PET/CT imaging studies were done at similar times using the same mouse model.
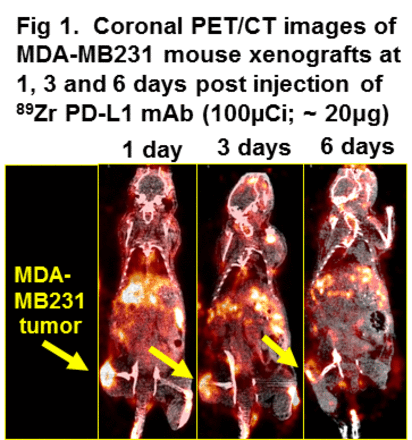
Results: ZPD exhibited high affinity (Kd ~ 0.3 nM) and detected moderate PD-L1 expression levels in MB cells which was comparable to published known levels. In MB xenografts ZPD distributed rapidly in tissue with the highest uptakes occurring in spleen (60 to 30%ID/g) and lymph nodes (LN; 19 to 27%ID/g) at all time points which would be expected as both have high levels of murine PD-L1+ immune cells. MB tumor uptakes (2.8 to 2.4 %ID/g) were lower than the spleen and LN indicating decreased PD-L1 expression which is consistent with the modest PD-L1 levels found in vitro in MB cells. From 1 to 7 d ZPD was highly retained in the MB tumors and LN while clearance was observed in all other tissues except femur. Femur uptakes increased by ~74% from 1 d to 7 d which most likely represents free 89Zr resulting from ZPD metabolism. Tissue to muscle ratios (T:M) for PD-L1+ tissues were highest for the spleen (48 to 30) over the 7 d with some decreases at later times while LN (14 to 32) and tumor (2 to 5) steadily increased from 1 to 7 d. In PD-L1 mAb dose escalation studies the spleen T:M were decreased (> 45%) when PD-L1 mAb doses were 蠅 +20 µg indicating that ZPD binding is PD-L1 specific and dose dependent (Table 1). In contrast LN and tumor T:Ms were increased 4 to 5 fold at 20 and 40 µg PD-L1 mAb doses (Table 1) mostly likely due to ZPD levels increasing in the blood (input) from PD-L1 mAb blockade of the spleen. From PET images MB tumors could be discerned as early as 1 d (Fig.1) and had tumor T:M of ~12 (3 d) reflective of the ZPD imaging dose (20 µg) and comparable to the > +20 µg PD-L1 mAb coinjections (Table 1).
Conclusion: ZPD exhibited specific and high affinity for PD-L1 in vitro and had target tissue uptakes correlating with PD-L1 expression levels in vivo. These results suggest that clinical ZPD PET imaging may have value in identifying patients who may benefit from PD-L1 immunotherapies. Further these data indicate that ZPD uptakes in PD-L1+ tumors maybe increased with appropriate PD-L1 mAb protein loading which would improve detection of PD-L1+ lesions in human subjects. Research Support: Molecular Imaging Program, NCI; NCI Contract No. HHSN261200800001E (Leidos Biomedical Research employees)
Table 1. Effects of PD-L1 mAb dose escalation on Zr-89 PD-L1 mAb Tissue:Muscle ratios