Abstract
The aim of this prospective study was to assess the predictive value of 18F-FDG PET/CT imaging for pathologic response to neoadjuvant chemotherapy (NACT) and outcome in inflammatory breast cancer (IBC) patients. Methods: Twenty-three consecutive patients (51 y ± 12.7) with newly diagnosed IBC, assessed by PET/CT at baseline (PET1), after the third course of NACT (PET2), and before surgery (PET3), were included. The patients were divided into 2 groups according to pathologic response as assessed by the Sataloff classification: pathologic complete response for complete responders (stage TA and NA or NB) and non–pathologic complete response for noncomplete responders (not stage A for tumor or not stage NA or NB for lymph nodes). In addition to maximum standardized uptake value (SUVmax) measurements, a global breast metabolic tumor volume (MTV) was delineated using a semiautomatic segmentation method. Changes in SUVmax and MTV between PET1 and PET2 (ΔSUV1–2; ΔMTV1–2) and PET1 and PET3 (ΔSUV1–3; ΔMTV1–3) were measured. Results: Mean SUVmax on PET1, PET2, and PET3 did not statistically differ between the 2 pathologic response groups. On receiver-operating-characteristic analysis, a 72% cutoff for ΔSUV1–3 provided the best performance to predict residual disease, with sensitivity, specificity, and accuracy of 61%, 80%, and 65%, respectively. On univariate analysis, the 72% cutoff for ΔSUV1–3 was the best predictor of distant metastasis-free survival (P = 0.05). On multivariate analysis, the 72% cutoff for ΔSUV1–3 was an independent predictor of distant metastasis-free survival (P = 0.01). Conclusion: Our results emphasize the good predictive value of change in SUVmax between baseline and before surgery to assess pathologic response and survival in IBC patients undergoing NACT.
Inflammatory breast cancer (IBC), the rarest and most deadly form of primary breast adenocarcinoma, is associated with a 5-y survival rate of about 40% (1). Distant metastases are frequently present at the time of diagnosis, and 18F-FDG PET/CT has been shown to be sensitive for the detection of metastases (2). The current consensus treatment consists of neoadjuvant chemotherapy (NACT) with an anthracycline- and taxane-based regimen, associated with trastuzumab for HER2-positive tumors, followed by mastectomy and axillary lymph node dissection for clinical responders and nonmetastatic patients, locoregional radiotherapy, and, when appropriate, endocrine therapy (3). Chemosensitivity may be the best prognostic indicator in IBC (4,5). Assessment of clinical response by tumor palpation is often inaccurate in IBC patients because of the presence of breast swelling and edema and a diffusely infiltrating behavior of the tumor without a measurable mass (5). Pathologic response is accurately assessed at final surgery. As in the case of non-IBC patients, IBC patients achieving a pathologic complete response (pCR) after NACT have longer disease-free and overall survival than patients with residual disease (6). Pathologic response to NACT in stage II and noninflammatory stage III breast cancer has been shown to be predicted by serial 18F-FDG PET/CT during treatment (7–10). In contrast to non-IBC, few data are available on the predictive value of 18F-FDG imaging for response to NACT in both metastatic and nonmetastatic IBC (11).
The aim of this study was to prospectively assess the predictive value of PET/CT imaging for non-pCR to NACT and prognosis in a homogeneous series of nonmetastatic IBC patients. The PET/CT criteria were quantitative parameters: maximum standardized uptake value (SUVmax) on PET1 (PET at baseline) and changes (Δ) in SUVmax and metabolic tumor volume (MTV) between PET1 and PET2 (PET after the third course of NACT) (ΔSUV1–2; ΔMTV1–2) and PET1 and PET3 (PET before surgery) (ΔSUV1–3; ΔMTV1–3).
MATERIALS AND METHODS
Patients
This study was part of a previous prospective study that assessed the value of 18F-FDG PET/CT in the initial staging of 59 consecutive women with unilateral IBC, staged T4d according to the classification of the American Joint Committee on Cancer (2,12). From April 2003 to June 2007, 23 of these women with newly diagnosed unilateral nonmetastatic IBC and treated by mastectomy with axillary lymph node dissection after NACT underwent 3 serial PET/CT scans. All patients received 6–8 courses of NACT with anthracycline (either fluorouracil, epirubicin, and cyclophosphamide or doxorubicin and cyclophosphamide) with or without docetaxel, 100 mg/m2, every 21 d. Five patients had HER2-positive tumors, but only 2 patients diagnosed after April 2005 received neoadjuvant trastuzumab. IBC patients underwent clinical examination, mammography, breast ultrasound, and image-guided core-needle biopsy, CT, or MR imaging. Tumor size was established by clinical examination and imaging. Exclusion criteria were age less than 18 y, previous breast surgery, chemotherapy or radiation therapy, inability to undergo serial PET/CT, ineligibility for surgery, and presence of distant metastases at diagnosis.
The institutional review board approved this study, and all subjects gave written informed consent.
Pathologic Response
At surgery, fresh surgical specimens were cut into 5-mm-thick slices and examined for macroscopic tumor. All pathology specimens were reviewed in a masked fashion by 2 pathologists. Pathologic response was assessed using the Sataloff classification (13). pCR was defined as the absence of invasive disease in the breast and axilla: stages TA and NA or stage NB. All other pathologic responses were classified as non-pCR.
Two groups of patients were then defined: the pCR group and the non-pCR group.
Clinical Response
Clinical response was assessed by palpation at each cycle and before surgery, according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.0) (14). Complete response was defined as tumor disappearance, and partial response as reduction of the tumor lesion by at least 30%.
PET/CT Imaging
Patients underwent PET/CT at baseline (PET1); after the third course of NACT, generally corresponding to midcourse (PET2); and before surgery (PET3) using the same scanner (Discovery LS; GE Healthcare). After the patients had fasted for at least 6 h, blood glucose levels were determined on capillary blood samples before 18F-FDG injection and were less than 7 mmol/L for all but 3 patients, who had blood glucose levels of 13.6, 12.7, and 10.6 mmol/L. Only 1 of these 3 patients was a known diabetic.
A 4–5 MBq/kg dose of 18F-FDG was injected intravenously in the arm opposite the breast cancer or via a dorsal pedal vein. Images were acquired approximately 60 min (73 ± 21 min) after injection in 2-dimensional mode from the skull to the mid thigh, with 5–7 bed positions of 4 min each. Patients were placed supine with the arms alongside the body and were allowed to breathe normally (shallow breathing) during PET and non–contrast-enhanced CT acquisitions. CT images were used for attenuation correction and fusion. Both attenuation-corrected and non–attenuation-corrected PET images, together with coregistered CT data, were reviewed.
PET/CT Analysis
PET/CT images were interpreted by 2 experienced nuclear medicine physicians, masked to the patients’ records.
SUVmax Measurements
A 3-dimensional region of interest was placed manually over the area of maximum activity on slices with the clearest definition of the entire breast tumor mass, skin, and locoregional lymph nodes. The highest initial SUVmax was measured on each PET/CT scan.
Relative Change in SUVmax (Normalized to 100% for PET1)
ΔSUV1–2 and ΔSUV1–3 were measured.
Global MTV
The MTV (cm3), including breast mass, skin abnormalities, and regional lymph node 18F-FDG uptake, was obtained by semiautomatic segmentation software, using volume delineation on the maximum-intensity-projection image. The corresponding extracted volume was obtained on the basis of an SUVmax cutoff of 2.5.
Relative changes in MTV between PET1 and PET2 (ΔMTV1–2) and PET1 and PET3 (ΔMTV1–3) were measured.
Statistical Analysis
The primary endpoint was residual disease. Nonparametric tests (Kruskal–Wallis, t test) were used for between-group comparisons.
The predictive performance of PET/CT for identification of responders and nonresponders was evaluated using receiver-operating-characteristic analysis (MEDCALC statistical software). The correlation between PET/CT and survival parameters was analyzed using the Kaplan–Meier method by univariate analysis. Overall survival and distant metastasis-free survival (DMFS) were calculated from the date of the baseline PET/CT scan.
The multivariate Cox proportional hazards model was used to assess the effects of multiple factors on overall survival and DMFS. The following factors were analyzed: decrease in tumor SUVmax, age, grade, hormone receptor (HR) status, and HER2 status.
All tests were 2-sided, and P values of 0.05 or less were considered statistically significant.
Analysis by HR and HER2 Status
The 3 main molecular subgroups of breast cancer (triple-negative, n = 7; HR-positive/HER2-negative, n = 11; HR-negative/HER2-positive, n = 5) were analyzed separately for SUVmax, ΔSUVmax, and survival parameters.
RESULTS
Patient Characteristics and Clinical and Pathologic Response
Patient, clinical, and pathologic characteristics and outcome are listed in Table 1. Mean age was 51 ± 12 y, and median follow-up was 76 ± 27 mo.
Clinical and Pathologic Characteristics and Outcome of 23 Patients with IBC
The overall pCR rate was 22% (4-stage TA NA and 1-stage TA NB). The pCR rate differed according to subtype, as 4 of the 5 pCRs were achieved in HR-negative tumors (Table 1). Only 1 pCR was observed among the 14 patients treated with an anthracycline alone, compared with 4 pCRs among the 9 patients who received anthracycline and docetaxel, associated with neoadjuvant trastuzumab in 2 patients (P = 0.018). No significant difference in pCR rate was observed between patients who received 6 courses of NACT and patients who received 8 courses.
A complete clinical response was noted in 5 patients (22%), associated with pCR in 3 patients (Table 2). No case of progressive disease was observed. No significant correlation was observed between pathologic and clinical response (P = 0.14).
Individual Characteristics of 23 IBC Patients
PET/CT Parameters and Pathologic Response
The median interval between PET1 and PET2 was 81 ± 18 d, and the median interval between PET2 and PET3 was 69 ± 21 d. The mean interval between PET3 and surgery was 20.5 d (median, 10 d; range, 1–114 d; surgery was delayed in 1 patient because of sepsis during chemotherapy).
PET1
PET1 showed increased 18F-FDG uptake in all primary tumors. Mean SUVmax on PET1 tended to be higher in the pCR group than in the non-pCR group, although this difference was not statistically significant (13.7 ± 5.7 vs. 9.5 ± 5.8, P = 0.18).
PET2 and PET3
Mean SUVmax did not significantly differ between the pCR and non-pCR groups on PET2 (4.3 ± 3 vs. 4.2 ± 3.2, P = 0.9) or PET3 (1.7 ± 0.5 vs. 2.5 ± 1.7, P = 0.14).
Mean Changes in SUVmax
ΔSUV1–2 did not significantly differ between the pCR and non-pCR groups (72% ± 16% vs. 54% ± 25%, P = 0.13). ΔSUV1–3 did not significantly differ between the 2 pathologic response groups but was higher in the pCR group (80.9% ± 6.4%) than in the non-pCR group (67.9% ± 19%, P = 0.08).
ROC Curves (Figs. 1–3)
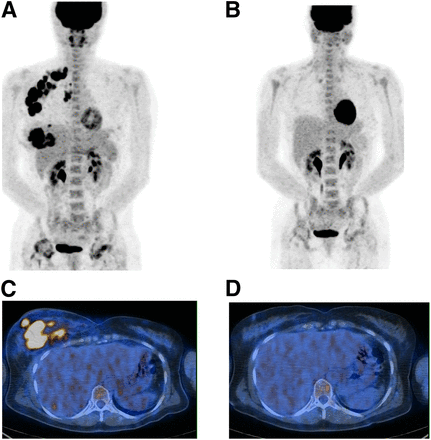
A 51-y-old woman with primary right IBC, axillary lymph node involvement, and pulmonary infection. IBC was grade 3 HR-negative/HER2-positive, with Ki-67 of 67.8%. (A and C) PET1 maximum-intensity projection image (A) and axial slice at level of breast (C). (B and D) PET2 maximum-intensity projection image (B) and axial slice at level of breast (D). ΔSUV1–2 and ΔSUV1–3 were −95%. Patient received 4 courses of anthracycline-based chemotherapy and 4 courses of taxane and trastuzumab. Pathologic complete response was found at surgery.
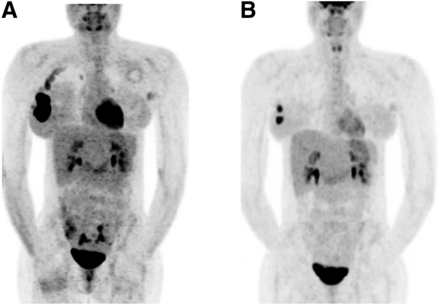
A 35-y-old woman with primary right IBC and affected axillary lymph node: PET1 (A) and PET3 (B) maximum-intensity projections. ΔSUV1–3 was −16.7%. IBC was grade 3 triple-negative, with Ki-67 of 30%. Patient received 6 courses of anthracycline-based chemotherapy. Nonpathologic complete response was found at surgery.
Capacity of ΔSUV 1–3 to predict residual tumor at surgery after completion of NACT, derived by area under receiver-operating-characteristic curve (0.75).
ΔSUV1–2 failed to predict residual disease, as no discriminant cutoff was identified. A 72% cutoff for the decrease in ΔSUV1–3 provided the best performance to predict pCR. The sensitivity for identification of residual disease (non-pCR) was 61%, and the specificity was 80%. Positive predictive value, negative predictive value, and accuracy were 92%, 36%, and 65%, respectively. According to this 72% cutoff, there were 11 good metabolic responders (>72%) (Fig. 1) and 12 poor metabolic responders (≤72%) (Fig. 2).
MTV
Mean MTV on PET1 and PET2 did not significantly differ between the pCR and non-pCR groups (t test). Mean MTV on PET3 was lower in the pCR group than in the non-pCR group, but the difference was not statistically significant (0.2 cm3 vs. 6.9 cm3; P = 0.13).
PET Analysis by Breast Cancer Subtype
SUVmax on PET1, ΔSUV1–2, and ΔSUV1–3 did not significantly differ among the 3 molecular subgroups. However, the lowest ΔSUV1–2 and ΔSUV1–3 were observed in the HR-positive/HER2-negative subgroup (for triple-negative tumors, 48.4% and 69% vs. 67.1% and 85% [P = 0.13 and 0.19], respectively; for HER2-positive tumors, 63.1% and 94% [P = 0.4 and 0.09], respectively).
PET/CT Parameters and Survival
ΔSUV1–2
An early change in SUVmax (ΔSUV1–2) was not associated with survival on univariate or multivariate analysis.
ΔSUV1–3
On univariate analysis, the 72% cutoff for ΔSUV1–3 was the best predictor of DMFS (P = 0.05). A trend was observed for prediction of overall survival (P = 0.17).
On multivariate analysis (Table 3), the 72% cutoff for ΔSUV1–3 was an independent predictor of DMFS (P = 0.01). Clinical response (P = 0.03) and tumor grade (P = 0.04) were also significantly associated with DMFS. None of the other possible confounders (pathologic response, age, HR status, or HER2 status) was significantly associated with survival after adjustment for the factors in the final survival model.
Multivariate Analysis for Distant Metastasis–Free Survival
A trend was observed with ΔSUV1–3 for prediction of overall survival (P = 0.17).
Survival by Breast Cancer Subtype
DMFS and overall survival were higher in the HR-positive/HER2-negative subgroup (70.2 and 94.3 mo, respectively) than in the triple-negative subgroup (36.7 and 63.7 mo [P = 0.08 and 0.09], respectively) or the HR-negative/HER2-positive subgroup (45.8 and 78.7 mo [P = 0.7 and 0.4], respectively) (Table 4).
Molecular Breast Cancer Subtype Analysis
DISCUSSION
IBC accounts for about 2% of all invasive breast carcinomas, with a metastasis rate at presentation of up to 30%. The present study included a small but well-defined population of 23 nonmetastatic IBC patients, all of whom underwent serial PET/CT and radical mastectomy. About one third of IBC patients are disease-free at 10 y; identification of the other two thirds of patients with poorer prognosis remains a crucial goal in this aggressive disease.
Prediction of response to NACT for IBC patients would be of considerable value, allowing the possibility of switching to another, more effective, regimen or targeted therapies in nonresponders. Response to chemotherapy in IBC patients is based mainly on clinical examination and has been correlated with survival (4). In non-IBC, conventional imaging to assess response to NACT has shown discordant results (15), probably because of the inability to differentiate fibrosis and granulomatous tissue from viable tumor cells. Volumetric MR imaging appears to be a more reliable tool to monitor response to NACT (16), but no published data on IBC are available. In the present study, comparison of PET/CT to morphologic imaging results was not performed, because patients had either CT or MR imaging at the initial work-up.
18F-FDG PET/CT has been used to assess response to NACT in non-IBC patients (7,10,17–22). Published data show a higher baseline 18F-FDG uptake in patients with pCR than in patients with a poorer response (7,17). Few data are available on this topic in IBC, although the value of 18F-FDG PET/CT in initial staging of IBC has been validated (2,12). In our series of IBC patients, baseline SUVmax was not predictive of residual disease; however, pCR was defined as complete or almost complete absence of invasive disease in the breast and lymph nodes.
Most studies performed in the neoadjuvant setting in non-IBC patients have assessed the absolute value of SUVmax after 1–3 cycles of NACT (7,10,18,19) and have suggested that 18F-FDG PET may accurately predict pCR. However, in our study, neither SUVmax on PET2 nor ΔSUV1–2 was predictive of residual disease, as can be explained in part by the presence of fibrosis, mucin pools, and foamy histiocytes during chemotherapy, perhaps more important in IBC, resulting in dilution of the 18F-FDG signal (20).
Several studies have also evaluated the performance of 18F-FDG PET/CT after completion of NACT to predict pCR (21,22) and found that 18F-FDG PET did not accurately assess residual tumor. Similar results were observed in the present study, as mean SUVmax on PET3 did not significantly differ between the pCR and non-pCR groups. In contrast, we found that the decrease in 18F-FDG uptake with a 72% cutoff for ΔSUV1–3 allowed identification of residual disease with high specificity.
Only a trend toward a correlation between MTV on PET3 and pathologic response was observed. Because it is often difficult to measure tumor volume, we tried to assess it by means of a global MTV, including breast, skin, and regional lymph node activity, delineated by semiautomatic segmentation based on a fixed SUVmax cutoff of 2.5. This global volume reflects the real tumor burden, including skin uptake, as, by definition, skin is involved in IBC and participates in breast 18F-FDG uptake. This MTV, based on a fixed SUVmax cutoff (23,24), has been shown to be highly reproducible (25). The use of this MTV remains controversial, but in the absence of a consensus, we decided to use this method.
Pathologic complete response to NACT is predictive of better survival, especially in HR-negative patients (6,26). pCR did not predict survival in this IBC series. However, the sample size was small, patients received various chemotherapy regimens, and the definition of pCR was stringent although the overall 22% pCR rate is in accordance with previous IBC series (6). Recent studies have suggested that the clinical objectives of 18F-FDG PET/CT and the criteria used to predict the efficacy of NACT should be established for separate molecular subgroups: estrogen receptor–positive and HER2-negative breast cancer, HER2-positive breast cancer, and triple-negative breast cancer (10,26,27). Eventually pCR to NACT seems to be a predictor of better survival, especially in HR-negative tumors, and response to NACT should be established for separate subgroups (6,26). Indeed, studies on IBC have demonstrated the presence of molecular subtypes similar to those of non-IBC but with overrepresentation of triple-negative and HER2-positive tumors (5,6,11). We analyzed separately these subtypes, but the limited size of each subgroup precludes definite conclusions. Nevertheless, in accordance with published data, we observed that patients with HR-positive tumors had better DMFS and overall survival than other subgroups despite a lower ΔSUV1–3 reflecting the poorer response to NACT (6,11).
The most significant finding of the present study is that contrary to pCR, ΔSUV1–3 with a 72% cutoff was an independent predictor of DMFS. This finding is consistent with a retrospective study in mostly metastatic IBC patients undergoing primary chemotherapy (11). This is a useful finding, as there is an urgent need to identify specific prognostic features in IBC. Although prognosis does not modify neoadjuvant management, the prognostic value of ΔSUV1–3 may be clinically useful. Several clinical trials are currently under way to develop treatment strategies for these patients with an unfavorable prognosis after NACT.
CONCLUSION
18F-FDG PET/CT appears to be useful to predict residual disease after NACT and survival in IBC. However, because of the small sample size, these results deserve further investigation in larger studies.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. No potential conflict of interest relevant to this article was reported.
Footnotes
↵* Contributed equally to this work.
Published online Jul. 9, 2015.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication March 25, 2015.
- Accepted for publication June 18, 2015.