Abstract
Studies report that 11C-flumazenil (FMZ) PET more specifically localizes the epileptogenic zone in patients with medically refractory focal epilepsy than 18F-FDG PET. However, practical aspects of 11C use limit clinical application. We report a phase I/IIa study assessing the clinical use of 18F-FMZ PET for the localization of the epileptogenic zone in patients with drug-resistant temporal lobe epilepsy (TLE). Receptor binding was quantified using kinetic modeling that did not require arterial sampling. Methods: Dynamic 18F-FMZ PET and static interictal 18F-FDG PET scans were compared in healthy controls (n = 17 for 18F-FMZ and n = 20 for 18F-FDG) and TLE patients with mesial temporal sclerosis on MR imaging (MTS, n = 12) and with normal MR imaging (NL TLE, n = 19). Masked visual assessment of images was undertaken. Parametric images of 18F-FMZ binding potential (BPND) were generated using the simplified reference tissue model. Region-of-interest analysis on coregistered MR images and statistical parametric mapping were used to quantify 18F-FMZ BPND and 18F-FDG uptake in the temporal lobe. Results: The visual assessment of static standardized uptake value images showed 18F-FMZ PET to have high specificity (16/17 [94%]) and moderate sensitivity (21/31 [68%]) for the localization of the epileptogenic zone, with a more restricted abnormality than 18F-FDG PET. However, the 18F-FMZ standardized uptake value images were falsely localizing in 3 of 31 patients (10%). Region-of-interest analysis demonstrated reductions in ipsilateral hippocampal 18F-FMZ BPND in patients with either MTS or NL TLE, compared with controls subjects. Ipsilateral hippocampal 18F-FMZ BPND was independent of both hippocampal volume and 18F-FDG uptake, whereas ipsilateral hippocampal volume was correlated with 18F-FDG uptake (r2 = 0.69, P < 0.0001). Statistical parametric mapping analysis demonstrated decreased uptake in 14 of 31 (45%) cases with 18F-FMZ PET and 18 of 29 (62%) with 18F-FDG PET. Cluster size was significantly smaller on 18F-FMZ than 18F-FDG images (37 vs. 160 voxels, P < 0.01). Conclusion: 18F-FMZ PET has potential as a clinical tool for the localization of the epileptogenic zone in the presurgical evaluation of drug-resistant TLE, providing information complementary to 18F-FDG PET, with a more restricted region of abnormality.
Temporal lobe epilepsy (TLE) is the most common form of drug-resistant epilepsy in adults. In patients with drug-resistant TLE, surgery is frequently the only therapeutic option with a realistic chance of rendering the patient seizure-free. One of the major goals of the presurgical evaluation is the identification of the site and extent of the epileptogenic zone. This is usually achieved by the concordance of complementary methods, in particular MR imaging and video-electroencephalographic monitoring, commonly supplemented by 18F-FDG PET or ictal SPECT (1). However, the area of abnormal metabolism identified by interictal 18F-FDG PET, or of hyperperfusion on ictal SPECT, often extends well beyond the epileptogenic zone across a large portion of the temporal lobe (and surrounding regions) (2,3). Furthermore, the localization rate of 18F-FDG PET in patients with extratemporal epilepsy is low (4), and obtaining true ictal SPECT injections is resource-intensive and logistically difficult for many centers. Therefore, the development and validation of new PET radiotracers that may more accurately identify the epileptogenic zone would be of great clinical value.
Decreased γ-aminobutyric acid A/central benzodiazepine receptor (GABAA/cBZR) density in association with the epileptogenic zone has been well described (5). Studies have reported that PET imaging using the GABAA/cBZR–specific radiotracer 11C-flumazenil (11C-FMZ) identifies a more restricted region of abnormality in the epileptogenic zone and has a higher sensitivity for extratemporal localizations (6,7). However, practical limitations of using 11C—in particular, its short 20-min half-life, necessitating an onsite cyclotron, and the need for arterial blood sampling (8) or multiinjection protocols (8,9) to model the tracer-binding characteristics—have prevented its integration into routine clinical practice. These limitations have motivated the development of 18F-radiolabeled FMZ conjugates for PET imaging (10,11). 18F has a significantly longer half-life than 11C, approximately 110 min, and a superior signal-to-noise ratio, making it more practical for routine clinical use (12). We have previously shown in rats that 18F-FMZ has suitable characteristics as a PET radiotracer, possessing a favorable metabolic profile and appropriate kinetics for modeling (10).
This study reports the use of 18F-FMZ PET in both patients with TLE and a group of well-characterized controls. This phase I/IIa trial aimed to provide proof-of-concept data for the clinical use of 18F-FMZ as a PET radiotracer for the localization of the epileptogenic zone in patients with drug-resistant TLE. The images were analyzed without the need for arterial blood sampling to enable the more practical application of this technology to clinical practice. The findings on 18F-FMZ PET were contrasted with those of 18F-FDG PET, the current best functional imaging technique for localizing the epileptogenic zone interictally in patients with drug-resistant TLE.
MATERIALS AND METHODS
Patient Population
Thirty-one patients with drug-resistant TLE (median age, 39 y; age range, 17–72 y; 11 men, 20 women) were studied. The patients were divided into the following 2 subgroups: well-localized unilateral TLE with concordant mesial temporal sclerosis and 18F-FDG hypometabolism (based on routine clinical reports) (MTS, n = 12) and well-localized unilateral TLE with concordant 18F-FDG hypometabolism without MTS or other structural lesion (NL, n = 19). All patients underwent at least 5 d of video-electroencephalographic monitoring to confirm the localization of the epileptogenic zone. For each patient, the determination of the localization of the epileptogenic zone was made at a meeting attended by at least 2 experienced epileptologists based on a concordance of all available clinical, video-electroencephalographic, imaging, and neuropsychologic data. Table 1 details the demographics and clinical features of the 3 participant groups.
Demographic and Clinical Characteristics of Study Groups
Two control groups were studied—17 healthy subjects (median age, 34 y; age range, 23–60 y; 8 men, 9 women) underwent MR imaging and 18F-FMZ PET, and a separate cohort of 20 healthy subjects underwent 18F-FDG PET (median age, 32 y; age range, 20–57 y; 10 men, 10 women). Information on the latter group was previously reported in a comparison with 18F-FDG uptake in epileptic subjects (3).
The study was approved by the Melbourne Health Human Ethics Research Committee (HREC #2007.286). All subjects provided written informed consent before participating in any study procedures.
PET Imaging
18F-FMZ PET and 18F-FDG PET scans were obtained at the Peter MacCallum Cancer Centre on the Discovery STE PET/CT scanner (GE Healthcare). The PET camera has bismuth germanate oxide detectors that acquire 47 contiguous slices with a thickness of 3.27 mm (axial length, 15.4 cm). Images were acquired in 3 dimensions, and the intrinsic axial resolution was 5.1 mm at a radius of 1 cm and 5.8 mm at a radius of 10 cm. The full width at half maximum of the scan was 5.4 mm. All studies were reconstructed into a 35-cm diameter, 128 × 128 transaxial matrix using the 3D VUE point (GE Healthcare) ordered-subset expectation maximization algorithm with 8 iterations, 8 subsets, and a gaussian postprocessing filter of 3.82 mm (full width at half maximum). A low-dose coacquired CT scan was used for attenuation correction.
18F-FMZ was prepared according to the method described by Ryzhikov et al. (13). Radiochemical purity was greater than 99%, and specific activity ranged from 125.06 to 480.63 GBq/μmol at the end of synthesis.
Subjects were intravenously injected with 18F-FMZ (4.23 MBq/kg; mean, 322 MBq; range, 197–481 MBq) via the left antecubital vein, and immediately a dynamic list-mode image of 1 h was acquired. Data were reframed into 10 × 30 s, 5 × 1 min, and 10 × 5 min frames.
Participants’ temperature, blood pressure, and oxygen saturation were monitored before and 90 min after injection of 18F-FMZ. Participants were also monitored for other signs of adverse effects on-site for 90 min after injection of 18F-FMZ and asked to report any ensuing adverse effects. Video monitoring during imaging and a postimaging interview were conducted to ensure patients did not experience a seizure during image acquisition.
Patients also underwent 18F-FDG PET as part of their routine clinical investigation (within 4 y of 18F-FMZ scan), as previously described (4). The electronic files for the 18F-FDG PET scans were unavailable for 2 NL patients and therefore were unable to be included in the analysis. All patients were imaged as outpatients in the interictal state. Patients fasted for 4 h before the scan and rested in a quiet, darkened room for 15 min before 18F-FDG administration and for at least 30 min afterward. 18F-FDG (250 MBq) was injected intravenously. Scanning commenced 60 min after radiotracer administration. Patients were scanned for 20 min in a single bed position, with the head positioned comfortably and immobilized with tape.
18F-FDG and FMZ PET/CT data were exported in Digital Imaging and Communications in Medicine P10 format for analysis.
Healthy control 18F-FDG PET images were obtained for a previous study (3).
MR Imaging
Participants were scanned on a 1.5-T clinical whole-body scanner (Sigma Horizon SE120; GE Healthcare) using a standardized protocol including a T1-weighted sagittal localizer, a coronal T1-weighted whole-brain volumetric series, axial and coronal T2- and fluid-attenuated inversion recovery (FLAIR)–weighted images, an oblique-coronal diffusion-weighted imaging (DWI) sequence, an oblique coronal T2 mapping sequence, and an oblique coronal echo-planar imaging (EPI) sequence. The whole-brain volumetric series was acquired using a fast spoiled gradient-echo technique with the following parameters: slice thickness, 1.5-mm; interslice gap, 0; repetition time/echo time, 11/2 ms pulse sequence; field of view, 22 × 22 cm; and matrix size, 256 × 256.
The criterion for classifying a patient as having unilateral MTS for the study was based on the clinical report by the neuroradiologist. This clinical diagnosis was made using standard visually assessed criteria, including hippocampal atrophy, altered signal (i.e., increased T2, decreased T1), and loss of internal architecture in the hippocampus (14).
Masked Visual Assessment of PET Images
Two experienced nuclear medicine physicians, masked to clinical data and imaging results, independently assessed all image sets. The first 5-min frame, corresponding to 10–15 min after injection, was used for the assessment of the static 18F-FMZ standardized uptake value (SUV) images and the static 18F-FDG images used. The image sets were presented in a random order, including both the 18F-FMZ and the 18F-FDG images from patients and controls as well as those from an additional 4 patients with other epilepsy syndromes so that the reviewers could not assume that the abnormal images localized to the temporal lobe. The reviewers were asked to localize the images to 1 of 8 sites (left or right; frontal, temporal, parietal, or occipital) or classify them as nonlocalizing. Reviewers were also asked to specify the level of confidence of their choice (nonlocalizing/lateralizing/probably localizing/definitely localizing) and to grade the extent and severity of the abnormality (mild to severe). If the 2 reviewers disagreed, a third masked reviewer was used. If this third reviewer classified the image localizing in agreement with 1 of the primary reviewers, the image was considered localizing, otherwise the image was considered nonlocalizing.
The results of the PET scans were assessed for sensitivity, specificity, and Cohen’s κ-scores of interobserver agreement. The extent and severity of the abnormality were quantified by weighting the severity of the abnormality across all regions affected (number of regions involved multiplied by the severity in that region: mild, 1; moderate, 2; and severe, 3).
Generation of Parametric Images of 18F-FMZ Binding Potential (BPND)
Parametric images of 18F-FMZ BPND were generated using PMOD software (PMOD Technologies Ltd.) and the simplified reference tissue model (SRTM) (15). The receptor-rich (left thalamus and left occipital cortex) and reference (pons) regions were delineated on the MR images coregistered to the PET data. Time–activity curves calculated for the 2 regions were used to calculate the set of basic functions.
Masked Visual Assessment of Parametric 18F-FMZ BPND Images
The 18F-FMZ BPND images were visually assessed in a masked manner, as described above for the static 18F-FMZ SUV images and 18F-FDG images, by the same 3 experienced nuclear medicine physicians.
Region-of-Interest (ROI) Analysis of Parametric 18F-FMZ BPND and Static 18F-FDG Images
After the generation of parametric 18F-FMZ BPND images, ROI analysis was performed on both static 18F-FDG images and parametric 18F-FMZ BPND images using Analyze (version 10.0; Mayo Clinic). ROIs for the hippocampi and anterior and posterior temporal lobes were delineated on the MR images manually by a single operator masked to the PET data. The hippocampi were delineated on coronal slices of the MR images, followed by adjustments in the sagittal plane. Anterior and posterior temporal lobes were delineated as previously described (3). These regions were then applied to the 18F-FDG and 18F-FMZ BPND images, from which 18F-FDG uptake and 18F-FMZ BPND values were derived. Additionally, the cerebellum was delineated and applied to 18F-FDG images for normalization of 18F-FDG uptake values (a second analysis using the pons for normalization of 18F-FDG uptake was performed; however, the resultant values were more variable than those derived using the cerebellum) (Supplemental Fig. 3; supplemental materials are available online only at http://jnm.snmjournals.org). These values were compared between hemispheres and against the control population to localize the epileptogenic zone. The 18F-FDG PET scans from the controls did not have matching MR images, so these scans were registered to an in-house MR template. However, 5 controls had to be excluded because of poor coregistration with the template MR images.
Statistical Parametric Mapping (SPM) Analysis of 18F-FMZ BPND and Static 18F-FDG Images
The PET and SPECT tool in SPM5 (Wellcome Institute of Neurology, UCL) was used. Participants’ images were normalized into the standard PET template provided in SPM5 using a 12-parameter affine transformation. Individual patient 18F-FMZ BPND and 18F-FDG images were compared with the control group for areas of increased and decreased 18F-FMZ BPND and 18F-FDG uptake. The criteria to consider a cluster of voxels significantly increased or decreased were a P value of less than 0.0005 and voxels greater than 20, based on previous work (3), a level at which no areas of reduced uptake occurred when individual control images were compared with the remainder of the control group images. The size of the resultant cluster of decreased 18F-FMZ BPND and 18F-FDG uptake was also measured.
Statistical Analysis
Statistical analyses were performed using Statistica (version 5.1; Statsoft Inc.). One-way ANOVA, the Fisher exact test, and the χ2 test were used as appropriate to assess demographics between patient groups. For ROI analysis, the Mann–Whitney U test was used to assess differences between controls and patients. The Wilcoxon rank-sum test was used to assess differences across hemispheres in patients and in cluster sizes after SPM analysis. Pearson correlation was used to assess relationships between variables. Data are expressed as median and interquartile range, and a P value of less than 0.05 was considered significant.
RESULTS
Tolerability and Adverse Effects
The 18F-FMZ injections were well tolerated, with no subjective or objective adverse effects detected. No seizures were reported by the patients or recorded by the staff during image acquisition.
Masked Review of PET Images
Figure 1 shows representative static 18F-FDG PET and 18F-FMZ SUV images used for masked visual inspection. On review of the 48 static 18F-FMZ images, there was agreement between the 2 primary reviewers for 37 images (77.1%), resulting in 11 images requiring assessment by the third reviewer. Interobserver agreement between the 2 primary reviewers was modest at a κ of 0.42. The third reviewer agreed with 1 of the primary reviewers in all but 6 cases. The images were classified as being localizing in 21 of 31 patients and 1 of 17 control subjects, resulting in a sensitivity of 67.7% and specificity of 94.1%; however, these results included 3 patients in the NL TLE group who were incorrectly localized.
Static 18F-FMZ PET and 18F-FDG PET coronal images used for masked visual review. (A) 18F-FDG PET image shows extensive hypometabolism throughout right temporal lobe (arrows). (B) 18F-FMZ PET image shows more restricted localization to mesial temporal region in same patient (arrows). (C) Symmetric 18F-FMZ distribution in control subject.
On review of the forty-eight 18F-FMZ BPND images, there was agreement between the 2 primary reviewers for 26 images (54.2%), resulting in 22 images requiring assessment by the third reviewer. Interobserver agreement between the 2 primary reviewers was low at a κ of 0.12. The third reviewer agreed with 1 of the primary reviewers in all but 4 cases. Correct localization of the epileptogenic zone occurred in 16 of 31 patients and all 17 images from the control subjects were classified as nonlocalizing, resulting in a sensitivity of 51.6% and specificity of 100%.
On review of the forty-nine 18F-FDG PET images, there was agreement between the 2 primary reviewers for 38 images (77.6%). Interobserver agreement between the 2 primary reviewers was modest at a κ of 0.60. The third reviewer agreed with 1 of the primary reviewers in all but 3 cases. Correct localization of the epileptogenic zone occurred in 21 of 29 patients, with all 20 control images being classified as nonlocalizing, resulting in a sensitivity of 72.4% and specificity of 100%.
Of the patients with MTS, 10 of 12 (83.3%) were correctly localized on the 18F-FMZ SUV images, compared with 8 of 19 (42.1%) for the NL group (P = 0.03, Fisher exact test, Table 2), with 18F-FMZ BPND images producing similar results, localizing 8 of 12 (66.6%) and 8 of 19 patients (42.1%), respectively (P = 0.27, Fisher exact test). On the 18F-FDG PET images, 11 of 12 MTS and 10 of 17 NL patients were correctly localized (P = 0.09, Fisher exact test). The differences in the localization rate were not significantly different between image sets (18F-FMZ SUV and 18F-FDG, P = 0.29; 18F-FMZ SUV and 18F-FMZ BPND, P = 0.80; 18F-FMZ BPND and 18F-FDG, P = 0.12; Fisher exact test).
Results of Masked Visual Review of Static 18F-FDG and 18F-FMZ SUV and BPND Images
The extent of decreased uptake was judged to be more restricted to the mesial temporal region on the 18F-FMZ PET images (median severity score, 1.5; range, 0–6) than on the 18F-FDG PET images (median severity score, 3; range, 0.5–12; P = 0.007; data not shown) (Figs. 1A and 1B).
ROI Analysis
Figure 2 shows the results of the ROI analyses of the parametric 18F-FMZ PET BPND and static 18F-FDG PET images (normalized to cerebellar uptake) and also the hippocampal volumes. Significant reductions were seen in 18F-FMZ BPND in the ipsilateral hippocampus in both patient groups when compared with both the controls and the contralateral hippocampus (Fig. 2A). On MR imaging, both the ipsilateral and the contralateral hippocampal volumes were significantly less in MTS and NL patients than in controls (Fig. 2B). In the MTS group, the ipsilateral hippocampi were significantly smaller than the contralateral hippocampi, whereas in the NL group the magnitude of the volume reductions was similar for both hippocampi. The normalized 18F-FDG PET uptake in the ipsilateral and contralateral hippocampal regions was significantly less in both patient groups than in controls (Fig. 2C). In the MTS patients, the relative 18F-FDG PET intensity was lower in the ipsilateral than in the contralateral hippocampal region but not in the NL group, where the 18F-FDG uptake was symmetrically reduced in both hippocampal ROIs.
Results of hippocampal ROI quantification for 18F-FMZ BPND (A), volumes on MR imaging (B), and 18F-FDG PET (C) intensity normalized to cerebellum. Significant reductions were observed in ipsilateral and contralateral hippocampi in both patient groups, compared with controls. Hypometabolism was also seen in ipsilateral hippocampus, compared with contralateral hippocampus in MTS group. *P < 0.05. **P < 0.01. ***P < 0.001. Mann–Whitney U test.
Supplemental Figure 1 shows 18F-FMZ BPND and 18F-FDG PET uptake for the anterior and posterior temporal lobe ROIs. There were no significant differences in 18F-FMZ BPND in the anterior temporal lobe between any of the participant groups. A trend to significance was observed in the ipsilateral anterior temporal lobe 18F-FMZ BPND between MTS patients and controls (2.39 vs. 3.06, P = 0.06; Supplemental Fig. 1A). In contrast, 18F-FDG PET uptake was significantly reduced in both patient groups, compared with controls in the anterior temporal pole (Supplemental Fig. 1B). This reduction was significantly more marked in the ipsilateral anterior temporal pole in the MTS group but was symmetric in the NL group. The volumes for the anterior temporal lobe ROIs based on the MR imaging did not differ significantly between patients and controls or between hemispheres in patients (data not shown).
There were no significant differences in 18F-FMZ BPND in the posterior temporal lobe ROIs (Supplemental Fig. 1C). There was no overall difference in the 18F-FDG PET intensities in the posterior temporal lobe ROIs between the patients and controls; however, there was an asymmetry in the MTS patients, being decreased on the ipsilateral side (Supplemental Fig. 1D). No significant differences were observed in posterior temporal lobe volume between groups or between hemispheres in patients (data not shown).
Supplemental Figure 2 shows 18F-FMZ BPND in the hippocampi and anterior and posterior temporal lobes, derived using the occipital cortex as the receptor-rich reference region. Direct comparison of hippocampal 18F-FMZ BPND values derived using the 2 reference regions showed no significant difference between the 2 techniques (Supplemental Fig. 2D, P > 0.05).
Supplemental Figure 3 shows ROI analysis of 18F-FDG uptake normalized to the pons. The asymmetry between the ipsilateral and contralateral hippocampi in the MTS group is maintained, but patient groups are no longer significantly different from the control values (because of greater variability in the control group) (Supplemental Fig. 3A). Direct comparison of hippocampal 18F-FDG uptake using the cerebellum and pons for normalization showed no significant difference in the patient groups but a significant difference in the controls.
Correlation Analyses
Figure 3 shows correlation analyses for the 18F-FMZ BPND, 18F-FDG PET, and MR images in the ipsilateral hippocampi of patients. Hippocampal 18F-FMZ BPND was not correlated with hippocampal volume (r2 = 0.15, P = 0.44, Fig. 3A) or 18F-FDG uptake (r2 = 0.19, P = 0.33, Fig. 3B). However, a significant correlation was observed between hippocampal 18F-FDG uptake and volume (r2 = 0.69, P < 0.0001, Fig. 3C).
Correlation between 18F-FMZ BPND, normalized 18F-FDG uptake, and MR imaging volumes in ipsilateral hippocampal ROI of patients. 18F-FMZ BPND was independent of both volume (A) and 18F-FDG uptake (B); however, 18F-FDG uptake and volume were highly correlated (C).
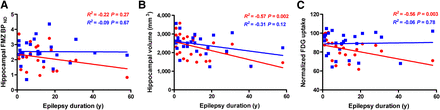
Figure 4 shows correlation analyses between the duration of the epilepsy and imaging measurements in the ipsilateral hippocampal ROI. No significant correlations were found between hippocampal 18F-FMZ BPND and epilepsy duration (Fig. 4A). However, ipsilateral hippocampal volume was found to correlate with epilepsy duration (r2 = −0.57, P = 0.002, Fig. 4B) as was ipsilateral hippocampal 18F-FDG uptake (r2 = −0.56, P = 0.003, Fig. 4C). No correlation was observed between 18F-FMZ BPND and time since last seizure (data not shown), in contrast to the findings of a previous study (16).
Correlation between epilepsy duration and 18F-FMZ BPND, volume, and 18F-FDG uptake in ipsilateral (red circles) and contralateral (blue squares) hippocampal ROI. (A) No relationship was observed between epilepsy duration and hippocampal 18F-FMZ BPND. Significant correlations were found between epilepsy duration and ipsilateral hippocampal volume (B) and normalized 18F-FDG uptake (C).
SPM Analyses
SPM analyses were performed for both parametric 18F-FMZ BPND and 18F-FDG PET images for all patients (Fig. 5; Supplemental Fig. 4). SPM analysis showed a cluster of voxels with reduced 18F-FMZ BPND in the region of the epileptogenic temporal lobe in 6 of 12 (50%) of MTS and 8 of 19 (42%) of NL patients (P = 1.00, Fisher exact test). SPM analysis of the 18F-FDG PET images showed a cluster of voxels with reduced metabolism in the epileptogenic temporal lobe in 10 of 12 (83%) of MTS and 8 of 17 (47%) of NL patients (P = 0.06, Fisher exact test). The cluster size in the epileptogenic temporal lobe was significantly smaller for the 18F-FMZ BPND than the 18F-FDG images (Fig. 5A, n = 11, P = 0.002), indicating a more topographically restricted region of abnormality. Consistent with this, SPM analyses of the 18F-FDG PET images showed a greater number of significant clusters in the posterior temporal lobe (P < 0.01) and a trend to more clusters in total (Fig. 5B, P = 0.08).
(A) Size (no. of voxels) of cluster localizing the epileptogenic zone is shown. 18F-FMZ cluster size was significantly less than 18F-FDG cluster size (P = 0.002) when comparing patients who localized on both images (n = 11). (B) Number of clusters identified after SPM analysis of both 18F-FMZ and 18F-FDG images, throughout brain (total); in mesial (mTL), anterior (ATL), and posterior temporal lobes (PTL); and in extratemporal (ET) region are shown. A trend to more clusters on 18F-FDG than 18F-FMZ was found (P = 0.08) and a significantly greater number of clusters identified in the posterior temporal lobe on 18F-FDG than 18F-FMZ (P < 0.01).
Surgical Outcome
Eleven patients underwent resective surgery, 6 from the MTS group and 5 from the NL group. In all cases, the histopathologic examination of the resected hippocampus confirmed the preoperative diagnosis of the presence or absence of MTS diagnosed on the basis of the MR imaging, defined by marked loss of neurons in CA1 and CA3 subfields and hilus with relative sparing of the CA2 and dentate regions (17). Five of the patients were seizure-free at the time of last follow-up (median follow-up, 11.5 mo; range, 2–25 mo). In the MTS group, 3 of 6 patients were seizure-free after surgery, compared with 2 of 5 in the NL group (P = 1.00, Fisher exact test). FMZ BPND, 18F-FDG uptake, volume ROI measures, or results of the SPM analysis were not predictive of surgical outcome (data not shown).
DISCUSSION
In a control population and TLE patients, this study assessed the use of 18F-FMZ PET in comparison to 18F-FDG for localizing an epileptogenic zone evaluating visual and quantitative analysis of imaging data, including SPM. The primary aim of the study was to generate proof-of-concept data regarding the use of this radiotracer for this purpose, establish that it produced high-quality PET image data and the feasibility of creating parametric BPND maps from the dynamic acquisition data using noninvasive modeling, and confirm safety and tolerability. The results of the 18F-FMZ imaging were compared with the current standard PET radiotracer for epilepsy evaluations, 18F-FDG. The study was not powered to demonstrate superiority or inferiority, compared with 18F-FDG PET, but rather provide a frame of reference that could be used to design subsequent larger comparative trials.
The study demonstrated that 18F-FMZ PET, analyzed without arterial blood sampling, provided high-quality images that specifically localized the epileptogenic zone in most MTS and NL TLE patients studied. The 18F-FMZ BPND decrease in the ipsilateral hippocampus was shown to be independent of reductions in hippocampal volume and 18F-FDG uptake. This finding agrees with several previous studies, which have shown that various 18F-FMZ binding parameters are independent of hippocampal volume (7,18,19).
In this study, we used a noninvasive method of kinetic modeling to generate parametric images of 18F-FMZ BPND—the SRTM (15). This methodology is robust (20), relatively simple to apply, and suitable for use in routine clinical practice. The use of the SRTM is further supported by a previous work by Odano et al. (12), which directly compared 18F-FMZ and 11C-FMZ in healthy volunteers. They showed no difference in uptake kinetics, metabolism, or measures of several binding parameters, including BPND, between the 2 radiotracers. Importantly, the study found no evidence of specific binding in the pons. Further, Odano et al. showed that BPND values derived from the SRTM were similar to those derived from traditional, invasive compartmental modeling and concluded that 18F-FMZ is a suitable alternative to 11C-FMZ for imaging GABAA/cBZRs in humans (12).
Consistent with previous reports using 11C-FMZ PET (7,21), we found in this study that 18F-FMZ PET images, whether analyzed visually or using quantitative measures (ROI or SPM), showed a more restricted region of abnormality than did 18F-FDG PET images in patients with drug-resistant TLE. 18F-FMZ abnormalities tended to be restricted to the hippocampus/mesial temporal region, compared with 18F-FDG hypometabolism, which extended into the surrounding temporal neocortex, agreeing with previous studies with 11C-FMZ (22,23).
The visual analysis of the 18F-FMZ PET SUV images had a higher localization rate than that for the parametric BPND images. However, the SUV images falsely localized the epileptogenic zone in 3 cases (Table 2), highlighting that localization by nonparametric SUV images should be interpreted with some caution, as has had previously been noted by others (20,24). The visual inspection of both the SUV and the BPND 18F-FMZ PET images had a lower localization sensitivity than that for 18F-FDG PET images. In contrast, previous studies with 11C-FMZ PET have generally reported a higher sensitivity than found in this study. Ryvlin et al. (7) reported that visual inspection of 11C-FMZ uptake images resulted in localization of the epileptogenic zone in 94% of TLE patients, including 100% of patients with unilateral MTS, 86% with normal MR imaging findings, and 66% with bilateral lesions. It is possible that our use of a reference tissue model (SRTM) to generate the parametric BPND images resulted in a lower localization sensitivity than if an arterial plasma input function method had been used (20). A future study that directly compares 11C-FMZ PET and 18F-FMZ PET images in the same patient population, using the same analysis methodology, is required to make definitive conclusions about the relative sensitivity of the 2 tracers for PET imaging in drug-resistant TLE. Of note, although all patients were selected on the basis of 18F-FDG hypometabolism being identified on clinical reading, which incorporated correlation with electroencephalography and MR imaging findings, masked reading of the 18F-FDG studies yielded a lower sensitivity (72.4%). Accordingly, 18F-FMZ scanning may also perform better in a clinical setting wherein more subtle abnormalities may be considered significant if concordant with other clinical findings.
A previous study investigating the use of SPM in analyzing 11C-FMZ PET in TLE patients found that SPM localized an ipsilateral decrease in 11C-FMZ BPND in 87% of patients. This rate was higher than that found when analyzing static images (80%) but lower than the detection rate when using an invasive model (volume of distribution, 100%) (20). The results of the current study did not attain this level of detection; however, the SPM analysis supported the results of the ROI and visual analyses, indicating that the changes in 18F-FMZ BPND are more restricted to the epileptogenic zone than those of hypometabolism on 18F-FDG PET. This was demonstrated by a smaller number and size of abnormal clusters that were focused in the mesial temporal region.
CONCLUSION
This study investigated the use of 18F-FMZ PET for the localization of the epileptogenic zone in patients with drug-resistant epilepsy. The results agree with previous 11C-FMZ studies, with decreases in 18F-FMZ BPND in the hippocampus, which are more marked on the epileptogenic side. The region of decreased 18F-FMZ BPND is more restricted than that of hypometabolism on 18F-FDG PET, indicating that it may provide a more specific localization of the epileptogenic zone. Further, the alterations in 18F-FMZ BPND were independent of both 18F-FDG uptake and volumes, whereas the latter 2 were correlated, indicating that 18F-FMZ may provide a different and complementary measure of the epileptogenic substrate to these established clinical assessments. Importantly, the 18F-FMZ BPND images could be constructed using a method that did not require arterial blood sampling, making it suitable for routine clinical practice. Overall, the results of this early-phase study support the need for subsequent larger studies to establish the potential of 18F-FMZ PET as a clinical tool in the presurgical evaluation of drug-resistant epilepsy—including extratemporal or atypical epilepsies—and to assess its incremental localizing value in comparison with 18F-FDG PET and MR imaging.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was funded by the Cooperative Research Centre for Biomedical Imaging Development Ltd. (CRCBID), Bundoora, Victoria, Australia, established and supported under the Australian Government’s Cooperative Research Centers program. Core academic partners of the CRC for Biomedical Imaging Development are the Australian Nuclear Science and Technology Organization (ANSTO), Menai, NSW; the Garvan Institute of Medical Research, Darlinghurst, NSW; Monash University, Clayton, VIC; and the Peter MacCallum Cancer Centre, Melbourne, VIC, all in Australia. Core commercial partners are Berthold Australia Pty Ltd., Bundoora, VIC; Cyclotek (Aust) Pty Ltd., Bundoora, VIC, Australia; and GE Healthcare Pty Ltd., Rydalmere, NSW, Australia. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Sue Belbin and Marian Todaro of the Department of Neurology, Royal Melbourne Hospital, for assistance with patient recruitment. We also thank Jason Callahan and the staff at The Centre for Molecular Imaging, Peter MacCallum Cancer Centre, for assistance with PET scanning.
Footnotes
Published online Jul. 15, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication April 16, 2012.
- Accepted for publication March 20, 2013.