Abstract
We examined prognostic interactions among cardiac autonomic function assessed by 123I-labeled metaiodobenzylguanidine (123I-MIBG) activity, hemoglobin, and kidney function in chronic heart failure patients. Anemia, chronic kidney disease, and impairment of cardiac sympathetic function have been shown as determinants of prognosis in heart failure patients, but there has been little information on their synergistic correlations with cardiac mortality. Methods: After evaluations of hemoglobin and estimated glomerular filtration rate (GFR), 468 heart failure patients with left ventricular ejection fraction less than 50% underwent cardiac 123I-MIBG imaging before discharge and were then followed up for a mean interval of 60.5 mo with a primary endpoint of cardiac death. Cardiac 123I-MIBG activity was quantified using heart-to-mediastinum ratio (HMR) and washout rate. Results: For 89 fatal cardiac events documented (19.0%), besides New York Heart Association class, multivariate Cox analysis revealed HMR, hemoglobin, and estimated GFR as significant independent determinants, with hazard ratios of 0.215 (P = 0.0129; 95% confidence interval [CI], 0.064–0.718), 0.821 (P = 0.0062; 95% CI, 0.708–0.946), and 0.984 (P = 0.0243; 95% CI, 0.970–0.998), respectively. Receiver-operating-characteristic analysis determined the thresholds for identifying patients at increased risk for cardiac death to be 1.57 for HMR, 11.9 g/dL for hemoglobin, and 46.4 mL/min/1.73 m2 for estimated GFR. Combining the 4 independent predictors incrementally (P < 0.05) improved prognostic powers maximally up to a global χ2 value of 97.3 compared with sole or other combinations. Conclusion: Hemoglobin, kidney function, and alterations of cardiac sympathetic nerve activity are independently and synergistically associated with increased cardiac mortality in chronic heart failure patients, together with New York Heart Association functional class.
Anemia has recently been noted as a prognostic marker and therapeutic target in patients with chronic heart failure. Anemia, however, is a common comorbidity of heart failure, and there are several causative clinical variables that could affect the prognosis of heart failure patients (1,2). Impaired kidney function or chronic kidney disease also has been recognized as a cardiovascular risk factor not only in patients without known cardiac diseases but also in heart failure patients (3). The autonomic nervous system is systemically stimulated in response to an anemia-induced decrease in oxygen delivery to peripheral tissues and is augmented more when heart failure coexists. Reduced kidney function is responsible for impairment of hemopoiesis and renal blood flow, leading to acceleration of the renin-angiotensin system, which could in turn exacerbate heart failure. These observations strongly suggest critical interactions of anemia and kidney dysfunction in heart failure patients, possibly magnifying the unfavorable effects of increased sympathetic activity on clinical outcomes (3). Cardiac sympathetic innervation and norepinephrine kinetics at nerve endings in failing hearts are noninvasively and quantitatively assessed using neuroimaging with 123I-labeled metaiodobenzylguanidine (123I-MIBG) (4). Sustained excess activation of cardiac sympathetic tone initially plays compensatory roles in patients with mild to moderate heart failure but decreases the efficiency of reuptake, turnover, and storage of norepinephrine at presynaptic endings in the myocardium (5). This process leads to an increase in norepinephrine concentration at the sympathetic cleft and desensitization of β-adrenoceptor, ultimately resulting in loss of norepinephrine content and impairment of sympathetic innervation at an advanced stage of heart failure. Increased norepinephrine spillover, decreased norepinephrine content at nerve endings, and sympathetic denervation in failing hearts can be revealed as an increase in 123I-MIBG washout rate from the heart or a decrease in 123I-MIBG activity. Several studies have demonstrated that abnormal 123I-MIBG kinetics are associated with lethal cardiac events in patients with moderate to severe heart failure (6–11). From these findings, we hypothesized that decreases in hemoglobin level and kidney function (estimated glomerular filtration rate [GFR]) increase the risk of cardiac mortality as assessed by cardiac 123I-MIBG activity in chronic heart failure patients. In the present study, we retrospectively analyzed prognostic data for 468 consecutive patients with symptomatic chronic heart failure and reduced left ventricular ejection fraction who had undergone cardiac 123I-MIBG imaging in combination with clinical evaluations, including evaluation of hemoglobin level and kidney and cardiac function.
MATERIALS AND METHODS
Patient Population
Five hundred one consecutive patients who had been admitted to our university hospital with symptomatic congestive heart failure and echocardiographic left ventricular ejection fraction less than 50% were enrolled in this study. For undetermined reasons, 33 patients (6.6%) were lost during the follow-up interval, and the prognostic data presented here were derived from the remaining 468 patients (93.4%) (340 men and 128 women; mean age, 62 y; range, 17–97 y). Congestive heart failure was diagnosed by the following clinical symptoms and signs according to the Framingham criteria: typical symptoms (palpitation, dyspnea, or orthopnea), neck vein distension, peripheral edema, lung rales, S3 gallop, and tachycardia together with chest radiography findings such as cardiomegaly, bilateral lung congestion, or pleural effusion. The diagnosis and etiology of heart failure were established at admission or thereafter using a 12-lead electrocardiogram, 2-dimensional or Doppler echocardiography, and, when necessary, stress perfusion imaging, coronary angiography, or CT for exclusion of noncardiac diseases showing similar symptoms or signs. We excluded patients with end-stage renal failure requiring dialysis therapy; patients with gastrointestinal or malignant disorders leading to anemia; patients who had undergone blood transfusion within the previous month; patients with insulin-dependent diabetes mellitus; patients with neurogenic disorders involving the autonomic nervous system; patients who had been treated with tricyclic antidepressant drugs, sympathomimetic agents, or other drugs that are known to interfere with cardiac 123I-MIBG uptake; and patients who were scheduled to undergo any cardiac surgery. After stabilization of clinical conditions after admission, patients underwent cardiac 123I-MIBG imaging and standard blood tests. Informed consent for registration in our database and use for clinical study was obtained in accordance with the guidelines of the ethics committee of our hospital. Table 1 shows the clinical backgrounds and the medications used; 128 (27.4%) of the patients had ischemic heart failure etiologies, and the remaining 340 (72.6%) had nonischemic heart failure etiologies.
Comparison of Clinical Data Between Groups With and Without Cardiac Events
Cardiac 123I-MIBG Imaging
Cardiac 123I-MIBG imaging was performed within 2 wk of the echocardiographic examination while the patients were hospitalized in stable clinical condition. 123I-MIBG (111 MBq) with a high specific activity was administered, and a standard imaging method used in our previous studies was applied (7,8). Briefly, cardiac planar and tomographic 123I-MIBG images of fasting, resting patients were obtained using a γ-camera equipped with a low-energy, general-purpose collimator 15–30 min (early image) and 4 h (late image) after an intravenous tracer injection. Cardiac 123I-MIBG activity was quantified as a heart-to-mediastinum ratio (HMR) by an experienced nuclear medicine technician unaware of the clinical data, who manually set a region of interest on the upper mediastinum and around the entire heart using an anterior-projection planar image. 123I-MIBG washout kinetics from the heart were calculated as washout rate using a polar-map technique with tomographic data because of the elimination of background activity. The high reproducibility of the quantitative method was confirmed in our previous studies (7–9).
Two-Dimensional Echocardiographic Examination
Standard 2-dimensional echocardiographic examinations were performed in our echocardiography laboratory by experienced cardiologists who were unaware of the clinical and scintigraphic data. Commercially available ultrasound machines equipped with a 2.5-MHz variable frequency transducer were used (SSH-160A, Toshiba; SSD760, Aloka; SONOS 2500, Hewlett-Packard; and Vivid 7, GE Healthcare). A 2-dimensional imaging mode was used to acquire apical 4-, 3-, and 2-chamber views while the patient was in the left lateral decubitus position. The left atrial dimension (mm) was measured by M-mode echocardiography. Left ventricular dimensions and wall thicknesses were measured, and then left ventricular ejection fraction was measured using the biplane modified Simpson method. Echocardiographic data obtained within 1 wk of 123I-MIBG imaging were used for analysis.
Blood Tests and Kidney Function Assessment
During cardiac 123I-MIBG imaging, blood was sampled from supine patients using an intravenous cannula for measurement of hemoglobin, serum sodium, and serum creatinine. Kidney function was evaluated by estimating GFR using the following formulas: estimated GFR = 194 × creatinine−1.094 × age−0.287, for men, and estimated GFR = 0.739 × male-estimated GFR, for women (12,13). Plasma brain natriuretic peptide (BNP) level was measured at the time of cardiac 123I-MIBG imaging or within 1 wk using a previously described standard technique (14). Briefly, samples for the BNP assay were transferred to chilled tubes containing aprotinin and immediately centrifuged, and then the concentration was measured by a specific immunoradiometric assay using a commercial kit. Because this study was retrospective, however, BNP data were available for only 267 (57.1%) of the 468 patients.
Follow-up Protocol
After discharge, all patients were examined at the outpatient clinic of our university hospital at least every 3 mo for a mean follow-up period of 60.5 mo by cardiologists who determined the necessity of blood tests, electrocardiography, chest radiography, echocardiography, or other examinations. The primary endpoint was all causes of cardiac death, consisting of pump failure death, arrhythmic death, and sudden cardiac death. Sudden cardiac death was defined as witnessed cardiac arrest and death within 1 h after onset of acute symptoms or unexpected death in patients known to have been well within the previous 24 h.
Statistics
Statistical values are presented as mean ± SD. Mean values were compared between the pump failure death group and the sudden cardiac death group using the unpaired t test, and prevalence was compared using the 2 × 2 table χ2 test. After univariate analysis, multivariate analysis with a Cox proportional hazards model was performed using the statistically appropriate number of significant variables identified by univariate analysis, which depended on the incidence of cardiac events. Receiver-operating-characteristic analysis was performed to determine the optimal cutoff for an independent significant parameter. Survival curves of patient subgroups were created by the Kaplan–Meier method to clarify the time-dependent, cumulative event-free rate and were compared using the log-rank test. For assessment of the incremental prognostic values of significant predictors, global χ2 values were calculated after the addition of several independent predictors identified by multivariate analysis, based on increases in the overall likelihood ratio. A P value of less than 0.05 was considered statistically significant. These analyses were performed using the SPSS statistical program package (version 11.0; SPSS Inc.).
RESULTS
During follow-up, primary cardiac events were documented in 89 patients (19.0%); 75 patients died of refractory pump failure and 14 of sudden cardiac death. There were 3 noncardiac deaths: 1 from hemorrhagic shock due to gastrointestinal bleeding, 1 from lung carcinoma, and 1 from rupture of a thoracic aortic aneurysm. The cardiac event group had a higher New York Heart Association (NYHA) class, less frequent dyslipidemia, lower hemoglobin level, greater creatinine and BNP levels, and lower estimated GFR than did the non–cardiac event group (Table 1). There was, however, no significant difference in other clinical or laboratory data between the 2 groups. Patients with cardiac events were treated more frequently with diuretics and nitrates but less frequently with statins than were patients without cardiac death.
There was no significant difference in any echocardiographic functional parameters between the 2 groups (Table 2). Compared with the non–cardiac event group, the cardiac event group had significantly less cardiac 123I-MIBG activity (early HMR, 1.77 ± 0.38 vs. 2.02 ± 0.55, P < 0.001; late HMR, 1.50 ± 0.36 vs. 1.81 ± 0.50, P < 0.001) and greater washout (41.3 ± 9.8% vs. 35.3 ± 11.3%, P < 0.001) (Table 3). Table 4 shows the results of univariate analysis using all variables and the results of Cox proportional hazards model analysis using the top 10 significant variables identified by the univariate analysis. Among the 10 variables, NYHA class, HMR of cardiac 123I-MIBG activity, dyslipidemia, use of nitrates, hemoglobin levels, and estimated GFR were significant independent predictors of cardiac death in the multivariate analysis. The χ2 values and hazard ratios were 7.75 and 1.423, respectively (95% confidence interval [CI], 1.121–1.827, P = 0.0054), for NYHA class; 6.18 and 0.215, respectively (95% CI, 0.064–0.718, P = 0.0129), for HMR; 7.48 and 0.821, respectively (95% CI, 0.708–0.946), for hemoglobin (P = 0.0062); and 5.08 and 0.984, respectively (95% CI, 0.970–0.998), for estimated GFR (P = 0.0243) (Table 4). Receiver-operating-characteristic analysis revealed optimal thresholds of HMR, hemoglobin level, and estimated GFR for identifying patients at greater risk of cardiac events: 1.57 for HMR; 11.9 g/dL for hemoglobin level, and 46.4 mL/min/1.73 m2 for estimated GFR (Fig. 1). The optimal sensitivity and specificity cutoffs were 70.8% and 62.8%, respectively, for an HMR of 1.57; 51.7% and 73.6%, respectively, for a hemoglobin level of 11.9 g/dL; and 58.4% and 66.0%, respectively, for an estimated GFR of 46.4 mL/min/1.73 m2. The event-free curves with adjustment for age, sex, diuretics, and nitrates for patients with HMR less than 1.57, hemoglobin level less than 11.9 g/dL, or estimated GFR less than 46.4 mL/min/1.73 m2 were significantly lower than those of patients without (Fig. 2). In the groups with versus without HMR less than 1.57, survival rates were 69.9% versus 92.6% (P < 0.001), respectively, at 48 mo and 62.8% versus 87.1% (P < 0.001), respectively, at 96 mo. In the groups with versus without hemoglobin level less than 11.9 g/dL, survival rates were 69.1% versus 89.8% (P < 0.001), respectively, at 48 mo and 60.9% versus 84.6% (P < 0.001), respectively, at 96 mo. In the groups with versus without estimated GFR less than 46.4 mL/min/1.73 m2, survival rates were 72.8% versus 89.8% (P < 0.001), respectively, at 48 mo and 60.9% versus 86.8% (P < 0.001), respectively, at 96 mo. When classified into 4 subgroups using HMR, hemoglobin level, and estimated GFR, there were significant differences in survival curves (Fig. 3). The subgroup with both HMR less than 1.57 and hemoglobin less than 11.9 g/dL or with both HMR less than 1.57 and estimated GFR less than 46.4 mL/min/1.73 m2 had the lowest survival rate among the subgroups. In contrast, the subgroup with both HMR of 1.57 or more and hemoglobin of 11.9 g/dL or more or with both HMR of 1.57 or more and estimated GFR of 46.4 mL/min/1.73 m2 or more had the highest survival rate among the subgroups. When all 4 independent predictors determined by Cox analysis were combined (i.e., NYHA class, hemoglobin, estimated GFR, and HMR), the prognostic power significantly increased maximally, with a χ2 value of 97.3 (Fig. 4).
Comparison of 2-Dimensional Echocardiographic Parameters Between Groups With and Without Cardiac Events
Comparison of Cardiac 123I-MIBG Kinetics Between Groups With and Without Cardiac Events
Results of Univariate and Multivariate Analyses in All Patients (n = 468)
Receiver-operating-characteristic analysis of late HMR of cardiac 123I-MIBG activity, hemoglobin, and estimated GFR, indicating that optimal cutoffs for identifying cardiac events are 1.57 for late HMR, 11.9 g/dL for hemoglobin, and 46.4 mL/min/1.73 m2 for estimated GFR. eGFR = estimated GFR; Hb = hemoglobin.
Event-free curves of 2 groups classified by cutoffs of 1.57 for late HMR, 11.9 g/dL for hemoglobin level, and 46.4 mL/min/1.73 m2 for estimated GFR after adjustment using NYHA functional class, dyslipidemia, and drug use. Patients with late HMR < 1.57, hemoglobin < 11.9 g/dL, or estimated GFR level < 46.4 mL/min/1.73 m2 (in red) had significantly lower event-free rates than did each counterpart (in blue). eGFR = estimated GFR; Hb = hemoglobin.
Event-free curves of 4 subgroups classified by cutoffs for both late HMR and hemoglobin (left) and by cutoffs for both late HMR and estimated GFR (right) after adjustment using NYHA class, dyslipidemia, and drug use. Patient subgroups with both late HMR < 1.57 and hemoglobin < 11.9 g/dL (in red) or with both HMR < 1.57 and estimated GFR < 46.4 mL/min/1.73 m2 (in red) had lowest survival rate among subgroups. eGFR = estimated GFR; Hb = hemoglobin.
Global χ2 values for predicting lethal cardiac events incrementally increase in combination with the 4 independent predictors; that is, NYHA functional class, hemoglobin, estimated GFR, and late HMR of cardiac 123I-MIBG activity. Predictive value is maximal when all 4 predictors are combined. eGFR = estimated GFR; Hb = hemoglobin.
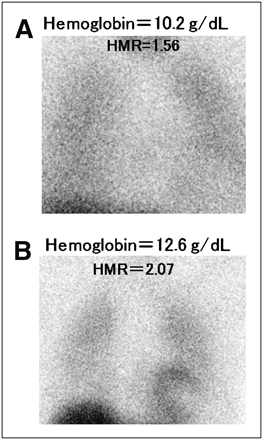
Despite the limited numbers of patients with BNP data (267) and cardiac events (44) in this population, a subanalysis was performed to clarify the prognostic value of BNP. Cox analysis using BNP and the previously identified 4 parameters (Fig. 4) revealed that, in addition to NYHA class and HMR, BNP level was a significant independent predictor of cardiac death, with a χ2 value of 3.85 and a hazard ratio of 1.0006 (95% CI, 1.0000–1.0012, P = 0.0497) when adjusted by hemoglobin level and estimated GFR (Table 5). Concerning the mode of cardiac death (Table 6), there was no significant difference in any variable, including echocardiographic and 123I-MIBG parameters, between the pump failure death and sudden cardiac death groups. Figure 5, however, shows that pump failure death was more frequently observed when hemoglobin was less than 11.9 g/dL and sudden cardiac death was more frequently observed when HMR was less than 1.57. Figure 6 shows typical cardiac planar images; one is from a 53-y-old man with an HMR of 1.56 and a hemoglobin level of 10.2 g/dL in whom cardiac death was documented; the other is from a 78-y-old woman with a nearly normal cardiac HMR (2.07) and hemoglobin level (12.6 g/dL) who had no cardiac event during follow-up.
Results of Univariate and Multivariate Analyses in Patients with BNP Data (n = 267)
Comparison of Clinical Data Between Pump Failure Death Group and Sudden Cardiac Death Group
Comparison of prevalence of pump failure death and sudden cardiac death. Pump failure death is observed more frequently when hemoglobin is <11.9 g/dL and sudden cardiac death is more frequent when HMR is <1.57. Hb = hemoglobin.
Planar 123I-MIBG images. (A) A 53-y-old man had markedly decreased HMR of 1.56 and reduced hemoglobin level of 10.2 g/dL, with NYHA functional class of III, estimated GFR of 27.1 mL/min/1.73 m2, left ventricular ejection fraction of 32.0%, and BNP of 469 pg/mL, and died of pump failure during follow-up. (B) A 78-y-old woman had both nearly normal HMR (2.07) and nearly normal hemoglobin level (12.6 g/dL), with NYHA functional class of I, estimated GFR of 54.3 mL/min/1.73 m2, and BNP of 67.8 pg/mL. Although left ventricular ejection fraction (32.0%) was reduced, she had no cardiac event during follow-up.
DISCUSSION
The present study demonstrated that NYHA class, hemoglobin level, estimated GFR, late HMR of cardiac 123I-MIBG activity, dyslipidemia, and use of nitrates are significant independent predictors of cardiac death. The study was not designed to reveal the clinical implications of dyslipidemia or use of nitrates. However, we noted that in chronic heart failure patients, impaired cardiac sympathetic innervation assessed by 123I-MIBG activity was synergistically associated with increased risk of cardiac death in combination with greater NYHA class and decreases in hemoglobin and kidney function.
Anemia and Heart Failure
Receiver-operating-characteristic analysis found that a hemoglobin cutoff of 11.9 g/dL identified a high-risk population of chronic heart failure patients but was not in itself a definition of anemia. In earlier studies, the prevalence of anemia in patients with chronic heart failure has ranged widely from 7% to almost 60% (1), probably because of differences in patient backgrounds and etiology and in the definition of anemia. Nevertheless, the hemoglobin level was nearly identical to the definition (12.0 g/dL) of anemia widely used in several heart failure studies (2) and to that of the National Kidney Foundation (15). Therefore, the hemoglobin level determined in this study has clinical implications for the detection of anemia, which is associated with a greater risk for cardiac death in heart failure patients. Several possibilities can be offered for the mechanism behind the cardiac risk of anemia. Anemia with a hemoglobin level of less than 13.0 g/dL is known to reduce renal blood flow and impair kidney function (16) and to increase venous return and cardiac workload (i.e., oxygen consumption) by stimulating sympathetic tone in response to a reduced oxygen supply, all of which result in the development and progression of left ventricular hypertrophy, remodeling, and myocardial ischemia, leading to fatal clinical outcomes.
Because this study was retrospective, the underlying causes of anemia were not determined and erythropoietin concentration was not measured. Impairment of kidney function likely plays a major role in decreasing the hemoglobin level, largely because of insufficient erythropoietin production. When GFR decreases to less than 60 mL/min, erythropoietin production and hemoglobin level linearly decrease (17); 190 (40.6%) of the 468 heart failure patients in this study had an estimated GFR of 50 mL/min or less. Chronic kidney disease is recognized not only as a comorbidity (with an incidence ranging from 20% to 40%) but also as a prognostic risk in heart failure patients (17,18). Some cardiac factors commonly seen in patients with moderate to severe chronic heart failure are at least partly responsible for reduced hemoglobin or anemia: persistent systemic congestion causes hemodilution (19), and malnutrition or cardiac cachexia disrupts iron absorption and the subsequent erythropoietic process (20). Independently of cardiac and kidney function, however, hemoglobin had a significant and additive prognostic value in this study, indicating that anemia plays a pivotal role in the occurrence of lethal cardiac events in chronic heart failure patients.
Kidney Function and Heart Failure
Impaired kidney function was also independently and incrementally associated with an increased risk for cardiac death in anemic patients with chronic heart failure. As is well recognized, chronic kidney disease or decreased GFR is related to increased cardiovascular events not only in patients without known cardiac diseases but also in heart failure patients. There are several possible explanations why impaired kidney function presents a cardiovascular risk to chronic heart failure patients. Simply, reduced GFR may be another aspect of the severity of chronic heart failure, which reduces renal blood flow, induces ischemia, and stimulates renin-angiotensin systems and sympathetic tone in the kidney. Impairment of kidney function suggests the presence of endothelial dysfunction and microvasculature damage, which commonly are underlying conditions and probably function as cumulative risks in cardiac–renal correlations (21). Decreased GFR could induce imbalances of water and electrolytes, leading to increases in volume overloading and arrhythmogenicity in heart failure patients. These cardiac–renal interactions possibly exacerbate patient prognosis via autonomic tone activation.
Impaired Cardiac Sympathetic Innervation and Heart Failure
This study clearly demonstrated a synergistic increase in the prognostic value of impaired cardiac 123I-MIBG activity in combination with hemoglobin and kidney function in chronic heart failure patients. This finding strongly suggests not only interactive mechanisms behind unfavorable outcomes in heart failure patients but also the clinical role of the combined assessment of these parameters for risk-stratifying heart failure patients and for selecting the therapeutic strategy. The study showed a higher likelihood of pump failure death when hemoglobin was less than 11.9 g/dL and of sudden cardiac death when HMR was less than 1.57, suggesting the need for a more aggressive prophylactic strategy, such as an implantable cardioverter defibrillator, against sudden cardiac death or lethal arrhythmias when cardiac sympathetic innervation is highly impaired.
Although the mechanisms have not been identified, a vicious cycle is likely to be generated by anemia, reduced estimated GFR, and impaired cardiac sympathetic innervation via hemodynamic and neurohumoral factors. Excessive increases in circulating catecholamines and sympathetic outflow have deteriorative effects on the myocardium and reduce production of neurotransmitters at nerve terminals (22). Anemia can exacerbate ischemia in failing hearts because of impaired high-energy phosphate production and increased oxygen consumption, resulting in impairment of the uptake-1 system of 123I-MIBG (norepinephrine) at nerve endings because sympathetic nerves are more susceptible to ischemia than are myocytes (23,24). It is well known that major cardiovascular events are mediated through the angiotensin II receptor. The renin-angiotensin system is activated by increased autonomic nerve function more excessively in anemic patients with heart failure and chronic kidney disease and may have nonhemodynamic effects on cardiac and renal structures by inducing cell injury and tissue fibrosis (25), whereas angiotensin II stimulates cardiac sympathetic function via the angiotensin II receptor at presynaptic sympathetic nerve terminals in the myocardium. Thus, lengthy and excessive stimulation of cardiac sympathetic function initially accelerates 123I-MIBG (norepinephrine) kinetics, with an increase in spillover or washout rate, and then induces downregulation of β-function (26–28), exhausting presynaptic innervation. Finally, neuron deficits shown by markedly reduced 123I-MIBG activity possibly occur, with decompensation of pump function and denervation supersensitivity (29) being responsible for lethal arrhythmic events.
Limitations
This was a retrospective and observational study using a clinical database in our laboratory. Despite consecutive enrollment based on inclusion criteria, selection bias cannot be completely ruled out because of the limited number of patients and because of single-center data for patients who had undergone cardiac 123I-MIBG imaging. There might have been other prognostic variables that were not analyzed in this study.
In particular, the presented results suggested unexpectedly that the use of dyslipidemia and statins plays a prognostic role in heart failure. The literature has shown that dyslipidemia and obesity may be related to a better prognosis in heart failure patients, but the concept is still questioned (30–33). Although observational and mechanistic studies suggest that statins benefit the prognosis of heart failure patients, larger randomized controlled trials have failed to demonstrate the expected benefits, and statin treatment in heart failure is also not determined in the clinical situation (33–35). Thus, further well-designed prospective studies are strongly needed to resolve the issue of dyslipidemia and statins in heart failure. Although the number of BNP data applicable for a multivariate analysis was limited, this study is likely to support our previous finding that BNP has significant prognostic efficacy independently of and in combination with 123I-MIBG data in heart failure (14). The findings suggest that BNP is superior to anemia or kidney dysfunction for risk-stratifying heart failure patients. In addition to studies evaluating the causes of anemia and kidney dysfunction, further studies are required to reveal the mechanisms of the accumulated risks for cardiac death. There is a need to establish clinical prophylactic strategies against anemia and kidney dysfunction in chronic heart failure patients at increased risk for cardiac death, as identified by cardiac 123I-MIBG activity (1). Finally, before cardiac 123I-MIBG imaging can become widespread in cardiology practice, standardization of quantitative 123I-MIBG assessment is needed (36,37), as is easy access to a radiotracer with a high cost-effectiveness. Recent metaanalyses (38,39) and a multicenter study using 123I-MIBG imaging (11) in Japan, Europe, and North America have demonstrated efficacy and feasibility in heart failure management.
CONCLUSION
In addition to NYHA class and kidney function impairment (reduced estimated GFR), hemoglobin and altered cardiac 123I-MIBG activity are independently and synergistically associated with cardiac mortality in chronic heart failure patients with systolic left ventricular dysfunction. Combined assessment of these variables can improve risk stratification of heart failure patients with reduced left ventricular ejection fraction for long-term cardiac death.
DISCLOSURE STATEMENT
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are particularly grateful to the cardiology staff of Sapporo Medical University School of Medicine for cooperation with clinical services. We also sincerely thank the staff of the Division of Nuclear Medicine and Radiology, Sapporo Medical University Hospital, Hokkaido Cardiovascular Hospital and Sapporo Cardiovascular Hospital for their technique assistance. No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Apr. 10, 2012.
- © 2012 by the Society of Nuclear Medicine, Inc.
REFERENCES
- Received for publication July 14, 2011.
- Accepted for publication January 3, 2012.