Abstract
When a therapeutic drug is competitively displaced at the binding sites of serum proteins, the free fraction of the drug will be increased, with an increase in the manifestation of pharmacologic properties. In the case of molecular imaging probes, total clearance and tissue distribution are increased in such circumstances. The aim of this study was to observe the increase in cerebral accumulation of N-isopropyl-p-123I-iodoamphetamine (123I-IMP) using the protein-binding displacement method with amino acid infusion. Methods: 123I-IMP binding to human serum was investigated and identified. In addition, protein-binding sites and the specific binding sites of human serum albumin (HSA) and α1-acid glycoprotein (AGP) were examined by ultrafiltration. Then, serum-binding sites and the displacement effects of amino acid infusion, including Proteamin 12X Injection and Kidomin, were confirmed in vitro. Subsequently, displacement of 123I-IMP serum protein binding with Proteamin amino acid infusion was tested in monkeys. A scintigraphic study of 123I-IMP in monkeys loaded with or without Proteamin was performed, and time–activity-curves of 123I-IMP brain accumulation in monkeys were evaluated. Results: 123I-IMP was bound to HSA site II and AGP to nearly equal extents. Compared with control conditions, loading with Proteamin and Kidomin markedly increased free fractions of binding site markers for HSA site II (14C-diazepam: 0.95% ± 0.04% for control, 1.40% ± 0.06% for Proteamin, 1.62% ± 0.05% for Kidomin) and AGP (3H-propranolol: 10.60% ± 0.32% for control, 13.18% ± 0.14% for Proteamin, 13.82% ± 0.72% for Kidomin). Amino acid infusions were thus suitable for use as displacers for binding site II and AGP. With use of Proteamin amino acid infusion to displace protein binding, the free fraction of 125I-IMP (14.95% ± 0.74%) was significantly increased in serum (19.24% ± 0.87%). In a 123I-IMP scintigraphic study of monkeys, average cerebral uptake in 2 monkeys increased by 1.34-fold with Proteamin. Our findings suggested that Proteamin treatment increased the free fraction of 123I-IMP, yielding rapid and pronounced cerebral accumulation in vivo. Conclusion: Amino acid infusion can improve brain accumulation by competitive displacement of serum protein binding in vivo. Further similar studies are needed with other radiopharmaceuticals.
It is generally accepted that only free drugs in serum exhibit pharmacologic activity. Coadministration of drugs with high protein-binding affinity may induce competitive displacement at specific binding sites and thus result in increased free drug concentration (1–3), with greater biologic activity of each drug than if either were administered alone. However, the increase in free drug concentration is often only transient, as a result of drug distribution and enhanced clearance.
Proteins bind a wide variety of drugs and thus significantly affect the pharmacokinetics and pharmacologic efficacy of such drugs. Contributions to drug binding in plasma include those of albumin, α1-acid glycoprotein (AGP), lipoproteins, and immunoglobulins (IgG). Several drugs bind with high affinity to one of the human serum albumin (HSA) sites and AGP (4,5). Sudlow et al. characterized 2 sites for drug binding, site I (also referred to as the warfarin-binding site) and site II (the benzodiazepine-binding site) of HSA (6). Although interaction of many drugs with AGP has been reported, the binding site of AGP has not been determined. It has recently been shown that AGP contains a wide drug-binding region for basic, acidic, and neutral drugs and that the binding sites on AGP are not completely separate and in fact partially overlap and influence one another (5,7).
Approximately 70% of N-isopropyl-p-123I-iodoamphetamine (123I-IMP, Fig. 1), a diagnostic agent for determination of regional cerebral blood flow in SPECT, is bound to serum proteins (8,9). If inhibitors with high protein-binding affinity could displace 123I-IMP serum protein binding, cerebral accumulation of this tracer would be increased. In fact, Kawai et al. reported an increase in 123I-IMP cerebral accumulation in monkeys using the protein-binding displacement method with 6MNA (a major metabolite of nabumetone) (10). We noted that an amino acid infusion could be administered more easily and safely than drugs. Amino acid infusions are widely used for parenteral nutrition and consist of water, glucose, salts, amino acids, vitamins, and sometimes emulsified fats. They are required for medical management of a variety of patients in a hypermetabolic state, as well as those who have neurologic disease, gastrointestinal disease, cancer, or psychiatric illness (11).
Chemical structure of 123I-IMP.
The purpose of this study was to investigate the displacement effects of 123I-IMP binding to 2 major human serum proteins, HSA and AGP. To identify the major binding proteins, we also evaluated the binding sites and effects of amino acid infusions on 123I-IMP serum protein binding to both HSA and AGP. In addition, we investigated whether cerebral accumulation of 123I-IMP was increased by amino acid infusion, using the competitive displacement method.
MATERIALS AND METHODS
Materials
Reagent-grade chemicals were used in this study. HSA (essentially fatty acid–free albumin), AGP, and human IgG were acquired from Sigma Chemical Co. Potassium warfarin, verapamil hydrochloride (Eisai), diazepam (Takeda Pharmaceutical Co., Ltd.), and propranolol hydrochloride (Dainippon Sumitomo Pharma Co., Ltd.) were also obtained. Amino acid infusions included Kidomin from Otsuka Pharmaceutical Factory, Inc., and Proteamin 12X Injection from Tanabe Seiyaku Co., Ltd. (Table 1). 14C-warfarin (2.1 MBq/mmol) was synthesized and purified by Amersham Biosciences, and 14C-diazepam (2.0 MBq/mmol) and 3H-propranolol (902.8 GBq/mmol) were synthesized and purified by Perkin-Elmer Life Sciences 123I-IMP (82.8 GBq/mmol) and 125I-IMP (10.5 GBq/mmol) (Nihon Medi-Physics) were used as molecular imaging probes. The radiochemical purities of the IMP and radiolabeled compounds were greater than 95.0%. Human pooled serum was prepared from blood samples obtained from 7 healthy male and female subjects. All subjects discontinued any medication for at least 5 d before blood sampling. The concentrations of albumin and AGP were determined to be 767 and 13 μM, respectively, using a COBAS Integra 400 Plus (Roche Diagnostics).
Content of Amino Acid Infusions
Identification of Protein Binding of 123I-IMP
Serum protein binding of 123I-IMP (111 MBq/mL) was measured by ultrafiltration (Ultracent-10; Tosoh). Centrifugal filter devices consisted of 2 compartments separated by a cellulose membrane, which had a molecular weight cutoff of 10,000 Da. 123I-IMP binding to human serum protein and to purified serum proteins such as HSA, AGP, and immunoglobulin (IgG) was examined. Mixtures (900 μL) of 123I-IMP at 220 kBq in 20 μL of saline and serum (or HSA, AGP, or IgG solution) were centrifuged at 3,000 rpm for 10 min at room temperature (model 5900; Kubota). The radioactivities (counts/20 μL) of the initial sample [I] and filtrate [F] were measured by a well-type scintillation counter (ARC-360; Aloka), and the free fraction and protein-binding rate were then determined using the following equations: Eq. 1
Eq. 1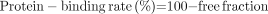 Eq. 2Human pooled sera were adjusted to an albumin concentration of 500 μM with phosphate buffer (pH 7.4). For use with purified human serum protein, solutions of HSA (740 μM, 4.9 g/100 mL of phosphate buffer), AGP (17 μM, 75.0 mg/100 mL of phosphate buffer), and IgG (62 μM, 1.0 g/100 mL of phosphate buffer) were prepared at a normal human serum concentration.
Eq. 2Human pooled sera were adjusted to an albumin concentration of 500 μM with phosphate buffer (pH 7.4). For use with purified human serum protein, solutions of HSA (740 μM, 4.9 g/100 mL of phosphate buffer), AGP (17 μM, 75.0 mg/100 mL of phosphate buffer), and IgG (62 μM, 1.0 g/100 mL of phosphate buffer) were prepared at a normal human serum concentration.
Examination of Specific Sites of Protein Binding by 123I-IMP on HSA and AGP
Effects of specific sites of protein binding by 123I-IMP on HSA and AGP were also examined in human pooled serum (albumin concentration, 500 μM) by ultrafiltration. Warfarin as a marker of HSA binding site I, diazepam as a marker of HSA binding site II, and propranolol and verapamil as markers of AGP were added to each serum sample at respective therapeutic concentrations (200 μM) before addition of the radioactive sample. The molar ratio of HSA to each drug was 46.9. Free fraction rates were obtained using Equation 1. The control experiment used saline as a substitute for site marker. Adsorption of drugs onto the membrane and apparatus was negligible (1,2,12).
Examinations of Specific Sites of Protein Binding by Amino Acid Infusions in Serum
The effects of amino acid infusion as a displacer were also examined in human serum (albumin concentration, 700 μM) by ultrafiltration. Proteamin and Kidomin were used because of their composition and content of amino acids. For confirmation of serum protein-binding sites of amino acid infusions, binding site markers such as 14C-warfarin (1.23 MBq/mL) and 14C-diazepam (3.7 MBq/mL) for binding sites I and II, respectively, and 3H-propranolol (37 MBq/mL) for AGP were used. Mixtures (200 μL) of binding site marker (5 μL at 7.4 kBq/mL), amino acid infusion (2 μL), and serum were centrifuged at 3,000 rpm for 10 min at 25°C (model 8800; Kubota). The molar ratios of HSA/L-tryptophan in Proteamin and HSA/L-tryptophan in Kidomin were 15.2 and 11.4, respectively. Radioactivities (counts/10 μL) of the initial sample [I] and filtrate [F] were measured with a liquid scintillation counter (LSC-5100; Aloka) after the addition of liquid scintillator (Clear-sol II; Nacalai Tesque) to scintillation vials. Free fraction rates were then determined using Equation 1.
Effects of Amino Acid Infusions on Binding of 123I-IMP
The effects of amino acid infusions as displacers on serum protein binding of 123I-IMP were examined. In this in vitro study, 125I-IMP (37 MBq/mL) was used instead of 123I-IMP. The free fraction of 125I-IMP was determined by ultrafiltration as described above, and free fractions were then calculated using Equation 1, with relative free fraction rates calculated as follows: Relative free fraction rate (fold) = free fraction of sample/free fraction of control. HSA concentration in human serum was adjusted to 700 μM with phosphate buffer (pH 7.4). The AGP concentration in human serum was also adjusted to 20 μM by adding AGP-purified serum protein, and 2-μL aliquots of amino acid infusion were added to 200 μL of a solution of 125I-IMP (1 kBq in 10 μL) and human serum. Normal saline solution was used as a control.
Displacement of 123I-IMP serum protein binding was attempted in monkeys with amino acid infusion. A scintigraphic study of 123I-IMP in 2 monkeys with or without loaded Proteamin was performed. Fasting Japanese monkeys (4.0 kg) were anesthetized with pentobarbital (12.5 mg/kg of body weight, intraperitoneally) after Ketalar (5.0 mg/kg of body weight, intramuscularly) and then received 123I-IMP (37 MBq in a 1.0-mL bolus via the left cephalic vein, intravenously). To avoid circulatory disorder, saline was infused via the right cephalic vein. Supplemental heat to maintain body temperature was provided by a warm blanket. The depth of anesthesia was monitored by observing the pupils and using pulse oximetry. The animals recovered from anesthesia 5 h after the imaging study and were then maintained in the normal fashion in our facility. Dynamic whole-body planar data were acquired every 1 min for 60 min with a 2-head scintillation camera (Prism 2000; Picker International). Subsequently, brain SPECT was performed over 30 min (30 s/frame) using a 3-head scintillation camera (Prism 3000; Picker International). For the displacement study, 5.0 mL of Proteamin (142.0 mg/kg of body weight, intravenously) was loaded just before the 123I-IMP injection. After 123I-IMP injection, Proteamin was also administered at 0.5 mL/min for 30 min (14.2 mg/kg of body weight/min, intravenously). All animal experiments were approved by the Ethics Committee of the University of Miyazaki.
Statistical Analysis
Data are expressed as mean ± SD. Groups were compared using the Student t test.
RESULTS
Determination of Protein Binding of 123I-IMP
Binding by 123I-IMP of human serum protein and purified serum protein was examined using ultrafiltration. In the in vitro binding assay shown in Figure 2, the percentage binding of 123I-IMP to human protein was 78.34% ± 0.86%. In addition, 123I-IMP bound moderately and almost equally to both HSA and AGP.
Protein binding of 123I-IMP to serum, HSA, AGP, and IgG at concentrations of 740, 17, and 62 μM, respectively. Each column represents mean ± SD of 3 independent experiments.
Examinations of Specific Protein-Binding Sites for 123I-IMP on HSA and AGP
Warfarin and diazepam are drugs that bind specifically to sites I and II, respectively, of HSA (7,13), whereas propranolol and verapamil bind specifically to AGP (14,15). Free fractions of 123I-IMP were significantly increased, compared with control, by the HSA site II marker diazepam and the AGP site marker propranolol (increases of 1.11- and 1.16-fold, respectively). There were no effects on binding rate of the HSA site I marker warfarin (Fig. 3). 123I-IMP thus appeared to bind site II on HSA and AGP.
Effects of binding site markers on 123I-IMP protein binding. Normal saline solution was used as control. Concentration of HSA in human serum was adjusted to 500 μM. Each site marker was loaded at 200 μM final concentration. Each column represents mean ± SD of 3 independent experiments. *P < 0.05.
Examinations of Specific Sites of Protein Binding by Amino Acid Infusions in Serum
As shown in Figure 4, Proteamin and Kidomin markedly increased not only the free fraction of 14C-diazepam (0.95% ± 0.04% for control, 1.40% ± 0.06% for Proteamin, and 1.62% ± 0.05% for Kidomin) but also that of 3H-propranolol (10.60% ± 0.32% for control, 13.18% ± 0.14% for Proteamin, and 13.82% ± 0.72% for Kidomin). 14C-diazepam concentrations in human pooled serum were, with Proteamin and Kidomin, increased by 1.47- and 1.71-fold, respectively, over the control level. The free fraction of 3H-propranolol was also increased (by 1.24- to 1.30-fold) over the control level. The free fraction of 14C-warfarin was reduced. These findings indicated that the 2 amino acid infusions we tested are suitable displacers for binding site II on HSA and AGP.
Free fraction rates of radiolabeled chemicals (14C-warfarin [A], 14C-diazepam [B], and 3H-propranolol [C]) loaded with Proteamin (PTA) and Kidomin (KDM) in normal human serum. Concentration of HSA in human serum was adjusted to 700 μM, and 2.0-μL aliquots of amino acid infusion were added to 200.0 μL of radiolabeled chemical–human serum mixture. *P < 0.02 vs. control. **P < 0.003 vs. control.
Effects of Amino Acid Infusion on Binding of 123I-IMP
We evaluated the relative free fraction rates of 125I-IMP with amino acid infusions as displacers of serum protein binding in vitro. The free fractions of 125I-IMP in human pooled serum with Proteamin and Kidomin were significantly increased to 1.29 and 1.12 times the control level, respectively (Table 2). 123I-IMP scintigraphy was therefore performed on 2 monkeys with or without Proteamin. Dynamic whole-body images and time–activity curves of 123I-IMP for each organ in monkeys are shown in Figures 5A and 6, respectively. Rapid cerebral accumulation was observed with Proteamin loading in both monkeys. The average cerebral uptake of 2 monkeys increased by 1.34-fold with Proteamin. Particularly, the plateaus of the time–activity curves were slightly shifted within 15 min with Proteamin loading in monkey 2. The plateaus of radioactivity in the brain of monkey 1 were significantly increased (1.44-fold), compared with the control condition, whereas relative changes in regional cerebral accumulation on brain SPECT (Fig. 5B) were not observed. These findings suggested that Proteamin treatment increased the free fraction of 123I-IMP and thus yielded rapid and pronounced cerebral accumulation in vivo.
123I-IMP whole-body scintigraphy (A) and brain SPECT (B) with or without Proteamin loading in same monkey (monkey 1). Proteamin (PTA; 5.0 mL) was loaded just before 123I-IMP injection. After 123I-IMP injection, Proteamin was also administered at 0.5 mL/min for 30 min.
Time–activity curves of 123I-IMP monkey 1 (A) and monkey 2 (B) on dynamic scintigraphy in same monkey. Findings with or without Proteamin (PTA) loading are shown for 123I-IMP accumulation in monkey.
Free Fraction of 125I-IMP and Relative Free Fraction Rate with Proteamin and Kidomin
DISCUSSION
Systemically administered drugs bind reversibly to serum proteins, and only free drugs in serum exhibit pharmacologic effects. The binding of drugs to proteins is an important factor inhibiting tissue distribution and excretion. The usefulness of protein-binding displacement has been reported. Takamura et al. suggested that coadministration of bucolome as a displacer of binding site I on HSA is effective in treating nephritic syndrome (16). 123I-IMP exhibits high protein binding. We therefore attempted to increase cerebral accumulation of 123I-IMP using the competitive displacement method with amino acid infusion, which is applied clinically for parenteral nutrition and easily administered to maintain high blood concentrations of parenteral formula.
In our in vitro binding assay, approximately 78% of 123I-IMP was bound to human serum protein, which has a high affinity to HSA site II (one of the specific binding sites of HSA) and AGP (Figs. 2 and 3). Yamasaki et al. have suggested that l-tryptophan in amino acid fluids specifically inhibits binding site II of HSA (12). Furthermore, we found that displacement by amino acid infusions might affect AGP and binding site II of HSA (Fig. 4). The amino acid infusions we tested are thus suitable for clinical use as displacers of binding site II of HSA and AGP.
These amino acid infusions were also found to have displacement effects on 125I-IMP in human pooled serum. The free fraction of 125I-IMP loaded with amino acid infusion, especially Proteamin, was significantly increased (1.29 times), compared with control (Table 2). Species differences were observed in displacement effects (10). A monkey study was then performed to confirm the displacement effects of Proteamin found in human serum studies, since a species similarity was expected between monkeys and humans. In the monkey scintigraphic study, rapid cerebral accumulation and a slight shift of time–activity curve plateaus were observed with Proteamin loading (Figs. 5 and 6). The radioactivity plateaus in brain were 1.24- and 1.44-fold higher in Proteamin-loading monkeys than those in control monkey 1 and control monkey 2, respectively. Kawai et al. reported that the combination of both binding inhibitors (of HSA site II and AGP) appears to exert a synergistic effect in displacement of IMP serum binding (10). It thus appeared that Proteamin treatment increased the free fraction of 123I-IMP and displaced 123I-IMP serum binding via a domino effect, yielding rapid and pronounced cerebral accumulation in vivo. The initial gradients of the brain accumulation curve with Proteamin loading were 1.3- and 1.4-fold higher in Proteamin-loading monkeys than in control monkey 1 and control monkey 2, respectively. We observed that the IMP uptake rate in the brain was increased just after administration. Furthermore, we observed different initial gradients in lung accumulation curves between Proteamin loading and control (Fig. 6). The free drug hypothesis assumes that only the free fraction of the drug in plasma can penetrate tissue and that drug transport in tissue is governed by the free drug concentration gradient between plasma and tissue, until equilibrium is reached (17,18). Thus, it appeared that Proteamin treatment increased the free 123I-IMP concentration gradient, with a tendency toward shift and a short time to peak value. However, the mechanism of IMP uptake into brain capillaries requires further study. These findings suggested the possibility of controlling the pharmacokinetics of 123I-IMP and imaging in clinical applications. Such a possibility could improve not only cerebral accumulation but also the signal-to-noise ratio of scintigraphic images. On the other hand, the possibility exists that reduced administration of 123I-IMP can yield image quality equal to that currently obtainable, with control of diagnostic costs. The displacement method could easily be applied to human studies under the conditions of the present examination. Displacement of radiopharmaceutical binding to serum proteins, we believe, provides a higher target organ accumulation and shortened imaging times, as well as higher signal-to-noise ratios in imaging.
CONCLUSION
This study showed that application of amino acid infusions as displacers of serum protein binding significantly increased the free fraction of IMP in vitro in serum and improved cerebral accumulation in monkey brain on whole-body dynamic scintigraphy. Amino acid infusions could thus be clinically useful for competitive displacement and regulation of the tissue distribution and kinetics of molecular imaging probes with high protein binding. Further similar studies are needed with other radiopharmaceuticals.
Acknowledgments
We thank Hideo Arita, Yasuyoshi Kuroiwa, Dr. Seigo Fujita, Dr. Takashi Jinnouchi, and the rest of the staff of the Department of Radiology, University of Miyazaki Hospital, as well as the members of the Kawai Laboratory of Kanazawa University, for technical assistance.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication November 11, 2008.
- Accepted for publication March 16, 2009.













