Abstract
Molecular imaging holds the promise of becoming a key diagnostic modality in cardiovascular medicine by allowing visualization of specific targets and pathways that precede or underlie changes in morphology, physiology, and function. As such, molecular imaging aims at detecting precursors or early stages of cardiovascular disease and at monitoring and guiding novel, increasingly specific and versatile cardiovascular therapies. Imaging of myocardial metabolism and autonomic innervation are already used in current practice, and a wide variety of other targets and probes is on the horizon. This focused review provides an overview of the opportunities and challenges that molecular imaging faces to fulfill its promises in clinical cardiovascular medicine.
The field of clinical cardiovascular imaging is changing. On the one hand, novel high-resolution techniques such as multislice CT or MRI provide ever-improving images of cardiac and coronary morphology. On the other hand, it becomes clear that the increasing variety and specificity of cardiovascular therapies require increasingly specific diagnostic information and that interrogation of morphology and pathophysiology may not suffice for this purpose (1–3).
Furthermore, whereas myocardial perfusion imaging is still the mainstay of cardiovascular radionuclide applications for the diagnostic and prognostic workup of coronary artery disease, several alternative imaging methodologies for noninvasive assessment of perfusion are emerging. At the same time, nuclear imaging technology has progressed significantly toward higher sensitivity and resolution, and an increasing number of molecule-targeted radiotracers is being introduced. These developments indicate an evolution of clinical nuclear imaging beyond the assessment of myocardial perfusion, toward characterization of molecular events on the tissue level. It is hoped that radiotracer techniques, with their unique translational potential and their superior detection sensitivity, will take a leading role in the emerging paradigm of personalized cardiovascular medicine, where therapeutic or preventive strategies are based on individual disease biology that is defined by molecular imaging tests (4).
CURRENT TRENDS IN CARDIOVASCULAR MEDICINE
Modern cardiovascular medicine on the one hand is continuously seeking to improve algorithms to prevent disease and disease complications. On the other hand, treatment of disease is becoming increasingly versatile and is being tailored to individual needs. The promise of cardiovascular molecular imaging is to expedite this trend by allowing visualization of specific targets and pathways that precede or underlie changes in morphology, physiology, and function, so that clinical decision making can occur at earlier stages of the disease process (Fig. 1). Rapidly advancing basic cardiovascular sciences, together with an increasingly successful convergence of preclinical research and imaging, have facilitated the introduction of novel therapeutic approaches to prevent and treat cardiovascular disease. The traditional options of pharmacotherapy, percutaneous intervention, or open-heart surgery are now augmented by various implantable devices, new pharmacologic agents, new surgical approaches, and targeted delivery of proteins, genes, cells, or tissue patches to diseased areas of the heart. Although these new therapeutic options are promising, selection of the most suitable candidates for a given approach requires diagnostic tests in order to optimize efficacy and cost-effectiveness and ultimately achieve broad clinical acceptance (5,6).
Continuum of coronary artery disease. Atherosclerosis progresses to ischemia and infarction and then to heart failure. Although conventional imaging describes extent and severity of these stages, molecular imaging aims at identifying precursors of disease development and progression. LV = left ventricular.
Given the increasing specificity of these novel therapies, it is likely that more specific imaging tests that interrogate cellular or subcellular target mechanisms will be needed. Decades ago, it was learned that assessment of left ventricular function and myocardial perfusion is not sufficient to select those patients with ischemic cardiomyopathy who will benefit from surgical revascularization. Assessment of myocardial metabolism, a tissue-specific biologic signal, has been found to be superior in this regard and gave rise to an entire new diagnostic field—that of myocardial viability imaging (7). It is the vision of cardiovascular molecular imaging professionals that, in a similar manner, several molecular imaging tests will emerge in the future as key diagnostic procedures for other novel clinical cardiovascular therapies.
CLINICAL CARDIOVASCULAR MOLECULAR IMAGING TECHNOLOGY
In the preclinical setting, molecular imaging of the cardiovascular system uses multiple modalities, including optical, nuclear, MR, CT, and ultrasound imaging (1,2), but clinical application in humans has been limited mostly to nuclear techniques. In addition to methodologic issues with the other techniques (optical imaging, for example, is confined to small animals because of limited penetration of light), the advantage of radionuclide techniques in humans is explained mostly by their superior sensitivity in detecting labeled molecules within the human body (2).
The roots of cardiovascular molecular imaging lie in the assessment of myocardial metabolism and myocardial autonomic innervation (Fig. 2). These areas have also emerged as its first true clinical applications. The Food and Drug Administration–approved glucose analog 18F-FDG is not used only for myocardial viability assessment. More recently, 18F-FDG has proven to be versatile and has successfully been used for imaging of atherosclerotic plaque inflammation (8) and labeling of stem cells for tracking after transplantation (6). Another 18F-FDG application may be ischemic memory imaging, in which fatty acid analogs such as 123I-β-methyl-iodophenylpentadecanoic acid have more recently also been introduced (9). For innervation imaging, the integrity of presynaptic sympathetic nerve terminals is measured using catecholamine analogs such as the recently Food and Drug Administration–approved 123I-metaiodobenzylguanidine or several available PET compounds (10). Risk stratification in heart failure has emerged as a promising application for innervation imaging, in which the degree of impaired innervation may provide more accurate information than conventional markers (11).
Schematic display of molecular imaging targets currently explored in clinical setting. BMIPP = β-methyl-iodophenylpentadecanoic acid; EPI = epinephrine; FFA = free fatty acids; FTHA = fluorothioheptadecanoic acid; HED = hydroxyephedrine; MIBG = metaiodobenzylguanidine; NET = norepinephrine transporter; TCA = tricarboxylic acid.
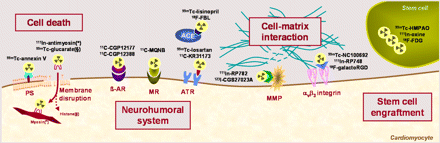
Other molecular mechanisms that have been explored in small human studies using multiple novel compounds include cell death (12), neurohumoral receptors (13), cell–matrix interaction (14,15), and stem cell tracking (Fig. 3) (6). The major purpose of these compounds can be described either as the detection of early disease stages that precede physiologic or morphologic abnormalities or as the guidance of therapy by more specific selection of suitable candidates and earlier response detection.
Schematic display of molecular imaging targets close to entering broader clinical application. (*) and (§) indicate different target molecules for cell death tracers: histones for glucarate (§) and myosin for antimyosin (*). ACE = angiotensin-converting enzyme; AR = adrenoceptor; ATR = angiotensin receptor; FBL = fluorobenzoyl-lisinopril; HMPAO = hexamethylpropyleneamine oxime; MMP = matrix metalloproteinase; MQNB = methylquinuclidinyl benzilate; MR = muscarinic receptor; PS = phosphatidylserine.
DETECTION OF EARLY DISEASE AND DISEASE PRECURSORS
For earlier detection of disease, molecular imaging techniques are primarily focusing on 2 areas: the vulnerable plaque, which precedes myocardial infarction, and left ventricular remodeling, which precedes heart failure.
Clinical observations that acute coronary events often result from rupture of atherosclerotic plaques at sites with no or minor luminal narrowing have stimulated the search for techniques to identify vulnerable, rupture-prone lesions (8). These may show characteristic morphologic features but may still differ in their biology and their activity, ultimately leading to rupture. Molecule-targeted approaches aim at plaque inflammation, apoptosis, extracellular matrix activation, or platelet binding. But many of the approaches are still at the preclinical or early clinical level, suggesting that this field remains a work in progress. Challenges related to the best targeting approach, to translation of animal model results to the clinical setting, to adequate imaging methodology for visualization of coronary artery biology, and to a suitable target patient population need to be overcome. The final goal of these efforts is improved clinical risk assessment through in vivo assessment of vascular biology.
Interestingly, many of the molecular mechanisms thought to play a role in plaque vulnerability also play a role in the development of left ventricular remodeling. These mechanisms include inflammation, apoptosis, matrix-metalloproteinase activation, and integrin expression (12,14,15). Activation of the renin-angiotensin system, impairment of autonomic innervation, and changes in substrate use are other relevant pathways that can be targeted for this purpose (10,13,16). Remodeling is defined as the continuous increase in left ventricular chamber size after a triggering event (mostly myocardial infarction), which ultimately leads to clinically overt contractile dysfunction and heart failure. Because of increasing survival rates for myocardial infarction, remodeling is an increasing health care problem if the substantial costs for treatment of heart failure are considered. A technique for predicting who is at high risk for remodeling and heart failure would therefore be of considerable value. Molecular imaging of the aforementioned targets holds the promise of better identifying individuals at risk, by identifying key biologic mechanisms that trigger the process. But despite the variety of available targets and tracers, conclusive evidence of clinical usefulness has not yet been obtained for these approaches. However, the goal may be somewhat easier to achieve in a clinical setting than through molecular identification of vulnerable plaques, because the myocardium is a larger target that can be imaged more robustly.
GUIDANCE AND MONITORING OF THERAPY
Although the use of clinical molecular imaging to detect earlier stages of disease and to guide algorithms to prevent disease progression is promising, it remains a vision and requires continuing preclinical, translational, and clinical research. Another major area in which molecular imaging has already been clinically successful is the monitoring of therapy. Characterization of novel approaches, selection of the most useful candidates, guidance of therapeutic decision making, and monitoring of the effects of therapy have all been accomplished in human studies.
Ideally, the characterization of a molecular disease mechanism by imaging is directly translated into a therapeutic strategy aiming at correction of the molecular abnormality. A practical example is the alteration of myocardial substrate use in heart failure. Metabolic imaging has suggested that a substrate switch from glucose to fatty acids contributes to impaired efficiency and promotes heart failure development (16). More recently, similar metabolic imaging techniques have demonstrated the beneficial effects of a metabolic modulator, trimetazidine. The agent improved ejection fraction and reversed metabolic impairments by lowering fatty acid use (17).
Heart failure has also emerged as a primary target for sympathetic neuronal imaging. Global downregulation of myocardial catecholamine uptake predicts deterioration because it seems to be involved in a vicious circle whereby impaired function leads to a hyperadrenergic state, which in turn leads to further impairment of function (10,11). Also, importantly, after myocardial infarction there seems to be a poorly innervated border zone of viable myocardium that results in electrophysiologic abnormalities and may be a substrate for life-threatening arrhythmia (18,19). This regional pattern could be used to better guide therapy directed at prevention of sudden cardiac death in the future.
Finally, some novel molecular and cellular interventions have been moving rapidly from preclinical to clinical settings and encountered difficulties in reproducing the success observed in animals when applied in the human setting. These difficulties have triggered the development of specific molecular imaging techniques to further elucidate the mechanisms and efficacy of these therapies. Key examples are cardiac gene therapy, for which reporter gene imaging methodology has been introduced (3,5,20), and cardiac stem cell therapy, for which multiple techniques for cell labeling to track their retention and engraftment have been introduced (6,21,22). Thus, although the specificity of novel cardiovascular therapies is continuously increasing, it is becoming increasingly obvious that a therapy targeting tissue-specific biologic pathways will also benefit from more specific diagnostic tests that allow for visualization of these pathways.
TRANSLATION OF NOVEL APPROACHES
Thus, there are significant opportunities and promises for molecular imaging in the practice of cardiovascular medicine. Although clinical application is currently limited mostly to imaging of metabolism and innervation, the challenge of translating preclinical compounds into the clinical arena must be overcome in order to broaden the clinical cardiovascular molecular imaging toolbox. This move into the clinic requires further research, facilitation of regulatory steps, and recruitment of funding. Importantly, the specificity of molecule-targeted approaches may limit their clinical application to specific situations and may narrow scientific and commercial interest in a new approach. It is therefore important to emphasize that many molecular mechanisms relevant to cardiac disease are also relevant to vascular disease and tumor biology, thus increasing the applicability of a targeted compound. Ultimately, the further translation, broadening application, and clinical success of cardiovascular molecular imaging will require a sustained, concerted effort by basic scientists, imagers, clinical cardiologists, professional societies, and industry.
CONCLUSION
Molecular imaging has great potential to contribute to clinical cardiovascular medicine by improving the understanding of disease processes and therapeutic mechanisms. It is hoped that this potential will result in algorithms for earlier disease detection and in improved therapeutic decision making. Metabolic imaging techniques already play a clinical role, and imaging of the autonomic nervous system is about to reach clinical acceptance. Finally, there is a plethora of other innovative tracers directed against molecular targets that are highly relevant to cardiovascular medicine. Translational progress is therefore highly relevant to broadening the clinical role.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication December 8, 2008.
- Accepted for publication January 14, 2009.