Abstract
For noninvasive monitoring of cellular status by dual reporters, a dual membrane protein reporter system was developed and its in vivo applicability was examined. Human sodium iodide symporter (hNIS) and mutant dopamine D2 receptor (D2R) transgenes were chosen considering their complementarity. Methods: pIRES-hNIS/D2R containing NIS and D2R linked with an internal ribosomal entry site (IRES) was constructed and transfected into human hepatoma SK-Hep1 and rat glioma C6 cells. The cell lines stably expressing hNIS and D2R (named SK-ND and C6-ND) were produced, which was confirmed by messenger RNA expression of reporter genes. The functional activities of hNIS and D2R were measured by 125I uptake assay and 3H-spiperone receptor-binding assays. A biodistribution study was performed on SK-ND tumor-bearing mice using 99mTc-pertechnetate and 3H-spiperone. In vivo hNIS expression was examined using 99mTc-pertechnetate γ-camera imaging and, D2R expression was examined using a 3H-spiperone autoradiographic study. Results: 125I uptake of SK-ND and C6-ND cell lines showed a maximum 97-fold and 43-fold increase, respectively, which were completely inhibited by KClO4. Specific 3H-spiperone binding to SK-ND and C6-ND cell homogenates was observed, which were completely inhibited by (+)-butaclamol. Among the dual reporter gene−expressing cell lines, the activities of both reporters were inversely correlated with each other. Competition assay of hNIS-expressing cells by D2R vector transfection and D2R-expressing cells by hNIS vector transfection showed a dose-dependent decrease of hNIS and D2R activities, respectively. In the biodistribution study, 99mTc-pertechnetate accumulated 10-fold and 3H-spiperone accumulated 4-fold more in SK-ND tumors than that in parental SK tumors. In vivo imaging of 99mTc-pertechnetate persisted until 5 wk after the cell graft in SK-ND tumors. Autoradiographic study of brain tissues from these mice also revealed an accumulation of 3H-spiperone in SK-ND tumors. Conclusion: We developed a dual membrane-bound positron and γ-imaging reporter system of hNIS and D2R. We observed its reporting capability in vitro and in vivo and elucidated that these 2 membrane protein reporters competed with each other in their expression. Although we expect that hNIS and D2R transgenes can complement each other as a dual reporter system, we suggest that one needs to validate the ratio of expression of the 2 membrane protein reporter transgenes for cellular status tracking.
The imaging techniques using reporter genes transfected to the cells are most appropriate for tracking the localization and survival of grafted cells in vivo in live animals. Among a variety of molecular imaging tracking techniques, the nuclear imaging method provides tomographic images as well as invaluable quantitative information for small or large animals and humans. This is compared with optical imaging, which cannot be applied to humans because of the lack of tomographic imaging capability associated with poor transmission from the deep tissues. Thus, development of reporter genes for nuclear imaging is required (1–4). Currently, dopamine D2 receptor (D2R), sodium iodide symporter (NIS), and herpes simplex virus 1-thymidine kinase (HSV1-tk) have been proposed as reporter genes for nuclear imaging (5–7). However, nuclear imaging is inevitably accompanied by background activity after injection of radionulides or radioligands. Normal physiologic radioligand distribution compromises the visibility of reporter-expressing grafted cells. A dual nuclear reporter might overcome these problems, and we chose 2 radionuclide reporter genes, human NIS (hNIS) and D2R, to track grafted cells.
NIS is a membrane-bound protein, which selectively transports iodide anion into thyroid cells. Its activity enables imaging with 99mTc-pertechnetate or 124I (8–10) or therapy with 188Re and 131I. 99mTc-Pertechnetate or 124I does not pass the blood–brain barrier so that NIS-expressing cells cannot be tracked in the brain region. In whole-body imaging, thyroid, stomach, and bladder could be considered as background in 99mTc-pertechnetate or 124I imaging while tracking the grafted cells. D2R is another membrane-bound protein, which is normally found in the striatum and pituitary. We can image this gene activity using 11C-raclopride; however, 11C-raclopride is normally taken up in the striatum, and background is high in the brain (11,12). Liang et al. reported that a D2R mutant had no effect on signal transduction even though the ligand binds to the target receptor (13). In this study, we established a dual reporter system to simultaneously express hNIS and the mutant D2R reporter genes linked with an internal ribosomal entry site (IRES) regulated by the same constitutive promoter (14). These 2 reporters produce membrane proteins and hNIS takes up 99mTc-pertechnetate and D2R binds 11C-raclopride (3H-spiperone). The aim of our study was to develop a dual reporter gene system for radionuclide imaging so that the shortcomings of each reporter gene might be overcome.
We examined the functional activities of these transgenes in various cell lines established from the same parental cell lines in vitro and their competitively expressive activities of 2 reporter membrane proteins; using 1 cell line among these cell lines, we further examined the functional activities of the dual reporter system in vivo to elucidate the imaging characteristics.
MATERIALS AND METHODS
Construction of pIRES-hNIS/D2R
The coding sequence fragment of hNIS cut by Xho I and Not I restriction enzymes was inserted, regulated by a cytomegalovirus (CMV) promoter, into MCS (multicloning site) A of the pIRES vector (Clontech) backbone. Mutant rat D2R with an artificial change from TCC (Ser) codon to GCC (Ala) was released by Xba I and Not I restriction enzymes from plasmid (kindly donated by Dr. Sanjiv Gambhir) (13). Released mutant D2R was placed in MCS B of the IRES so that hNIS and mutant D2R were linked to both ends of the IRES (pIRES-hNIS/D2R; Fig. 1A).
Schematic diagram of pIRES-hNIS/D2R vector construct. This plasmid was constructed with hNIS and D2R, which were linked with IRES under control of a CMV promoter. Neomycin resistance gene was placed downstream of simian virus 40 (SV40) promoter separated from a CMV promoter in the same vector. kb = kilobases.
Establishment of Cell Lines Expressing hNIS and D2R
SK-Hep1, a human heaptocarcinoma cell line, and C6, a rat glioma cell line, were cultured in RPMI medium (Jeil Biotechservices Inc.) containing 10% fetal bovine serum (Invitrogen Co.), 10 U/mL penicillin (Invitrogen), and 10 μg/mL streptomycin (Invitrogen). Purified recombinant plasmid (pIRES-hNIS/D2R) was transfected to SK-Hep1 with lipofectamine. SK-ND (SK-Hep1-hNIS/D2R) stably expressing hNIS and D2R was established by selection with 100–1,000 μg/mL with geneticin (Invitrogen) for 2 wk. C6-ND (C6-hNIS/D2R) was established by the same procedure. SK-Hep1 cells transfected only with pCMV-hNIS (SK-N) or transfected only with pCMV-D2R (SK-D) was also established by selection with hygromycin for 2 wk (Invitrogen).
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) Analysis for hNIS and D2R Genes
Total RNA was prepared from cultured SK-Hep1, C6, SK-ND, and C6-ND cell lines using Trizol (Invitrogen). Approximately 1 μg/mL of total RNA were reverse transcribed using Superscript II reverse transcriptase into complementary DNA. The PCR was followed using the specific primers listed below as templates. Amplified products were analyzed by ethidium bromide–stained agarose gel electrophoresis. Expression levels of both hNIS and D2R were calculated by dividing the intensity of each of the bands for hNIS and D2R by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for normalization. The DNA sequences of the specific primers for the hNIS, D2R, and GAPDH were as follows: hNIS: forward primer (5′-GCTAAGTGGCTTCTGGGTTG), reverse primer (5′-GTAAGCACAGGCCAGGAAAA); D2R: forward primer (5′-ACTGGTAATGCCGTGGG), reverse primer (5′-GCAATGATACACTCATTCT); GAPDH: forward primer (5′-ACCAGGGCTGCTTTTAACTCT), reverse primer (5′-GAGTCCTTCCACGATACCAAA).
125I Uptake Assay for hNIS Transgene Activity
SK-ND or C6-ND or other control cells (1 × 105 cells) were plated in a 24-well plate and cultured for 24 h. The iodine uptake was determined by incubating cells with 500 μL of Hanks' balanced salt solution (HBSS) containing 0.5% bovine serum albumin with 3.7 kBq of carrier-free Na125I and 10 μM NaI for 30 min. After incubation, the cells were washed twice, as quickly as possible, with 2 mL ice-cold HBSS buffer (15,16). The cells were dissociated by trypsinization, and the radioactivity was measured by a NaI well counter (Cobra II; Canberra Packard). A BCA (bicinchoninic acid) protein quantification kit (Bio-Rad Laboratories) was used to quantify the protein for calibration.
D2R-Binding Assay for D2R Transgene Activity
The cultured SK-ND cell lines were washed with phosphate-buffered saline (PBS, pH 7.2) and detached using a scraper. The harvested cells were lysed in 50 mM Tris buffer (pH 7.5) containing 150 mM NaCl and 1 mM ethylenediaminetetraacetic acid by sonication, and then the cell lysates were diluted to 100 μg/mL of total protein with assay solution (5 mM Tris-HCl, 150 mM NaCl, 0.025% ascorbic acid, and 0.001% bovine serum albumin, pH 7.4). Samples were separated into 2 groups. One group of samples was treated with 10 μM (+)-butaclamol (Sigma) on ice for 20 min and the other was not treated; both groups were then incubated with 16 nM 3H-spiperone (specific activity, 469.9 GBq/mmol [12.7 Ci/mmol]) at 4°C for 30 min. Samples were filtered through 25-mm GF/F glass microfiber filters (Whatman International Ltd.). Filters were immediately washed 3 times with 5 mL of washing solution (50 mM Tris-HCl and 150 mM NaCl, pH 7.4) and placed in scintillation vials containing 10 mL of scintillation fluid for 6 h. Radioactivity was measured using a liquid scintillation analyzer (Packard Bio Co.). A Scatchard plot analysis was performed to calculate the density (picomoles) of D2Rs per milligram of cellular proteins.
Linearity of Transgene Activities with Cell Numbers and Competitive Expression of Transgenes
125I uptake measured as counts per minute was converted to picomoles as determined by NaI molar concentrations. For the linearity study of hNIS and D2R expression, cells were plated in the wells by doubling the number and incubated until reaching a subconfluent density, and the transgene activities were counted per each well. To compare the activities of the 2 transgenes in the 6 cell clones, 125I uptake was again converted to picomoles per cell number (105 cells), and specific 3H-spiperone binding was expressed per milligram protein of cell homogenates.
We further performed the transient transfection of pCMV-D2R vectors in the concentration range of 0.1–1.6 μg into the SK-N cell line, which was stably expressing hNIS reporter gene. SK-Hep1 stably expressing luciferase reporter gene, which is a cytosolic protein, was used as a negative control (SK-Fluc). On the contrary, the SK-D cell line, which was stably expressing D2R reporter gene, was transfected with pCMV-hNIS vectors in DNA concentrations of 0.1–1.6 μg. SK-Fluc was also used as a negative control. After these transient transfections, 125I uptake and 3H-spiperone binding of SK-N and SK-D cells were examined.
Biodistribution Studies of hNIS and D2R in Nude Mice
Male BALB/c nude mice were xenografted with 1 × 106 cells of SK-ND and SK-Hep1 by subcutaneous injection. The tumors were allowed to grow for 2 wk until their mass reached <300 mg. 99mTc-Pertechnetate (74 kBq/0.1 mL) for hNIS and 3H-spiperone (74 kBq/0.1 mL) for D2R were administered to nude mice via the tail vein, and these mice (n = 4/group) were sacrificed at 1, 6, and 24 h after injection. Blood, tumor, and other organs were rapidly separated, weighed, and counted in a NaI well counter and liquid scintillation analyzer. The biodistributions of tumors and tissues were represented as a percentage of the injected dose per gram (%ID/g) of tissue (17). Animal studies were carried out in compliance with our institutional regulations.
Ex Vivo Autoradiographic Study for D2R Binding in Grafted SK-ND Tumors
The tumor or mouse brain tissues were removed and prepared as 10-μm sections using a cryostat microtome (Leica CM 1800). Sections mounted onto microscopic slide glasses were separated into 2 groups: total binding and nonspecific binding groups. The tissue sections in the total binding group were incubated with 4 nM 3H-spiperone and 1 μM ketanserine (to reduce nonspecific binding of 3H-spiperone onto the 5-hydroxytryptamine 2 [5-HT2] receptor) in 50 mM Tris-HCl buffer (supplemented with 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, and 1 mM MgCl2 × 6H2O, pH 7.4) for 1 h. Nonspecific binding was determined in Tris-HCl buffer containing 4 nM 3H-spiperone and 1 μM ketanserine in the presence of 1 μM (+)-butaclamol. The slide glasses were washed 2 times for 3 min in ice-cold 50 mM Tris-HCl buffer (pH 7.5) and quickly soaked in ice-cold distilled water for 10 s. Sections were dried in a stream of cold air. For autoradiographic experiments, imaging plates (Fujifilm) were exposed to slide glasses and were measured using a BAS-2000 Fujifilm (FLA-2000).
In Vivo 99mTc-Pertechnetate Imaging in Nude Mice with Subcutaneous Xenografts
BALB/c nude mice were xenografted with SK-ND and SK-Hep1 cell lines by subcutaneous injection: SK-Hep1, left shoulder (1 × 105 cells); SK-ND, right shoulder (1 × 105 cells) and right thigh (1 × 107 cells). The tumors were allowed to grow for 1, 2, and 5 wk. Scintillation images were acquired at each time using 99mTc-pertechnetate. General anesthesia was induced by intraperitoneal injection of 300 μL of ketamine and xylazine (2:1) solution. Thirty minutes after injection of 14.8 MBq of 99mTc-pertechnetate via the tail veins, the mice were placed in a spread prone position, and images were taken using a γ-camera (ON 410; Ohio Nuclear) equipped with a pinhole collimator.
RESULTS
Stable Transfection and Transgene Expression of hNIS and D2R in Cell Lines on RT-PCR Analysis
From SK-Hep1 and C6 parental cells and also from stable SK-ND and C6-ND clones transfected with pIRES-hNIS/D2R vector, total RNA was isolated, and on RT-PCR analysis the messenger RNA (mRNA) expression of transgenes was confirmed using hNIS, D2R and GAPDH primers (Fig. 2A). No mRNA of hNIS and D2R were expressed in nontransfected SK-Hep1 or C6 cells.
(A) Electrophoresis result from RT-PCR analysis of hNIS and D2R in both SK-Hep1 and C6 cells and their hNIS/D2R-transfected cell lines (SK-ND and C6-ND). On RT-PCR columns, mRNA expression of hNIS and D2R was observed in SK-ND and C6-ND cell lines, whereas neither gene was expressed in original nontransfected SK-Hep1 and C6 cells. (B) 125I uptake measured in SK-Hep1 and C6 cells and their hNIS/D2R-transfected cell lines expressing hNIS activity. SK-ND and C6-ND cell lines showed 97-fold and 43-fold higher 125I uptake than that of parental cells, respectively (black bars). 125I uptake of SK-ND and SK-N cell lines expressing only hNIS was almost similar to each other. 125I uptake of SK-ND, SK-N, or C6-ND cell lines was completely inhibited by KClO4, a specific NIS inhibitor (white bars). (C) Specific binding of 3H-spiperone for D2R measured in SK-Hep1 and C6 cells and their hNIS/D2R-transfected cell lines expressing D2R. SK-ND and C6-ND cell lines showed 8-fold higher specific binding of 3H-spiperone than that of parental cells. However, specific binding of SK-ND cell lines was 7-fold less than that of SK-D cell lines expressing only D2R.
Functional hNIS Activities in SK-ND and C6-ND Clones Measured by 125I Uptake in Vitro
The 125I uptake assay was performed in selected clones of the several stable clones of SK-ND and C6-ND. The SK-N clone transfected only with hNIS was included as a positive control. 125I uptake was 97-fold in SK-ND cell lines and 43-fold higher in C6-ND cells compared with that of the respective parental cells. 125I uptake in the SK-N clone was similar to that in the SK-ND clone. This 125I uptake was completely inhibited with 30 μM KCIO4, a competitive inhibitor of NIS (Fig. 2B).
D2R-Binding Activities in SK-ND and C6-ND Clones in Vitro
In the same clones, 3H-spiperone binding activity was measured in cell homogenates from SK-ND and C6-ND clones, and the SK-D clone transfected only with D2R was included as a positive control. An approximately 8-fold increase in specific 3H-spiperone binding was observed in both SK-ND and C6-ND cell lines. In the SK-D clone, in which D2R transgene is regulated directly by a CMV promoter without the IRES downstream effect, specific 3H-spiperone binding was 7-fold higher than that in SK-ND cell lines. No receptor-specific 3H-spiperone binding was observed in SK-Hep1 cells (Fig. 2C).
Linearity of Transgene Activities with Cell Number and Competitive Expression of hNIS and D2R in Vitro
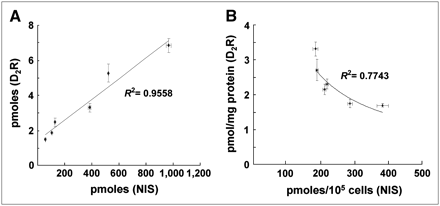
The SK-ND clone was seeded by doubling the cell density from 1 × 104 to 3.2 × 105, and the hNIS activity and 3H-spiperone binding in cell lysates of wells showed a proportional increase as the cell number increased (Fig. 3A). Among the SK-ND clones, 6 stable clones showing functional activities of hNIS and D2R transgene expression were chosen. 125I uptake per cell number (105 cells) and specific 3H-spiperone binding per milligram of protein of cell homogenates showed an asymptotic inverse correlation (Fig. 3B).
(A) Correlation of functional activities of hNIS and D2R represented by total 125I uptake and total 3H-spiperone binding in wells containing doubling numbers of SK-ND cells. As cell number increased, activities of hNIS and D2R increased proportionally in a linear fashion. The square of the correlation coefficient was 0.95. (B) Functional activity of 2 membrane-bound reporter proteins (hNIS and D2R) showed asymptotic inverse correlation in 6 highly expressing SK-ND clones. Upon nonlinear curve fitting, the square of the correlation coefficient was 0.77. All data are expressed as mean ± SD of triplicate wells.
125I uptake in SK-N clones decreased gradually after transient transfection with the D2R vector as the concentration of D2R vector increased (Fig. 4A). Specific binding of 3H-spiperone to D2R in SK-D clones decreased after transient transfection with hNIS vector as the concentration of hNIS vector increased (Fig. 4B). Transfection of SK-N or SK-D cells with luciferase vector (cytosolic reporter) affected least the expression of hNIS in SK-N or D2R in SK-D cell lines.
Competitive effect between expression of 2 membrane-bound protein reporter transgenes. (A) SK-Hep1 cells stably expressing hNIS gene (SK-N) were further transfected transiently with pCMV-D2R vector at increasing concentration (0.1–1.6 μg). Firefly luciferase (Fluc) expression vector was used as negative control. hNIS activity in SK-N cells gradually decreased as concentration of CMV-D2R vector increased. (B) SK-Hep1 cells stably expressing D2R gene (SK-D) were further transfected transiently with CMV-hNIS vector at increasing concentration (0.1–1.6 μg). D2R activity in SK-D cells gradually decreased as hNIS gene concentration increased. Neither NIS nor D2R activity was affected by Fluc vector transfection and expression of cytosolic luciferase protein.
Biodistribution of hNIS and D2R Activities of Grafted SK-ND Cells in Nude Mice
After 99mTc-pertechnetate or 3H-spiperone was injected into the tail veins, the mice were sacrificed at 1, 6, and 24 h later. The %ID/g of 99mTc-pertechnetate and 3H-spiperone in the tissues of the nude mice were plotted at each time point of 1, 6, and 24 h after injection (Figs. 5A and 5B). The 99mTc-pertechnetate uptake of the SK-ND tumor was 10-fold higher at 1 h than that of the SK-Hep1 tumor (Table 1). The accumulation of 3H-spiperone in the SK-ND tumor was 2.7-fold higher at 1 h than that of the SK-Hep1 tumor.
Biodistribution data of respective 99mTc-pertechnetate and 3H-spiperone uptake in SK-ND–bearing nude mice. (A) SK-ND tumors were subcutaneously grafted into the right hindlimb of 4 nude mice. After grafted SK-ND tumors had grown for 2 wk and 1, 6, and 24 h after injection of 99mTc-pertechnetate, tumors and various organs were dissected and weighed and their radioactivity were measured to yield %ID/g at given time points. (B) Tumor-bearing mice were sacrificed after intravenous 3H-spiperone administration. Tumors and the organs, including brain, were collected to measure accumulated radioactivity.
Ratio of SK-ND Tumor–Expressing hNIS and D2R to SK-Hep1 Tumor Control in Biodistribution
Ex Vivo Autoradiographic Experiment in Grafted SK-ND Tumor Cells in Mice
The tumor tissue and brains were isolated from the nude mice with grafted SK-ND cells for 3 wk. When the tumor tissue and brains were treated with 3H-spiperone, dense activity was observed on autoradiographic analysis (Figs. 6A and 6C) compared with the adjacent slices treated with both of 3H-spiperone and (+)-butaclamol (Figs. 6B and 6D). Specific binding of 3H-spiperone to tumor tissues was quite similar to the binding of 3H-spiperone to the striatum.
Autoradiographic images of SK-ND tumor and rat brain using 3H-spiperone. After SK-ND tumors were isolated from nude mice, in vitro binding assay was performed on these tumor slices. (+)-Butaclamol was used as D2R-specific inhibitor. Specific accumulation of 3H-spiperone was observed in SK-ND tumors (A) compared with that of the same tumors pretreated with (+)-butaclamol (B). When rat brain tissue was used as positive control for the same procedure, high specific binding of 3H-spiperone was shown in striatum (C) compared with nonspecific binding (D). D2R binding was inhibited using (+)-butaclamol in rat brain tissue (D).
In Vivo γ-Camera Imaging of hNIS Activity of Grafted SK-ND Cells in Mice
SK-ND cells (1 × 105 and 1 × 107 cells) and SK-Hep1 cells (1 × 105 cells) were grafted onto the right forelimb and hindlimb and the left forelimb. Whole-body planar scintigraphic images showed 99mTc-pertechnetate uptake in the grafted SK-ND cells at 1, 2, and 5 wk (Fig. 7). At 1 wk, 99mTc-pertechnetate uptake within the grafted regions of interest of the SK-ND tumor of 1 × 105 cells (right forelimb) was 2.5 times higher than that of the SK-Hep1 tumor (left forelimb). This ratio of SK-ND tumor increased from 4.0 at 2 wk to 7.7 at 5 wk. At 1 wk, 99mTc-pertechnetate uptake was prominent (7.8 times) at the SK-ND tumor of 1 × 107 cells (right hindlimb) compared with that of the SK-Hep1 tumor. At 2 wk, the tumor uptake of these SK-ND cells increased to 8.6 and then changed to 7.1 at 5 wk. However, the total count of the larger mass was increased by 11.3 times.
Serial whole-body prone scintigraphic images of nude mouse grafted with SK-ND at 1, 2, and 5 wk after graft using 99mTc-pertechnetate. Different cell numbers (1 × 105 and 1 × 107 cells) of SK-ND were inoculated into fore- and hindlimbs of right side, and 1 × 105 SK-Hep1 cells were grafted into left forelimb. Images were taken 30 min after injection of 99mTc-pertechnetate. 99mTc uptake was found on SK-ND tumor of right hindlimb (1 × 107 cells) at 1 wk and became visible on right forelimb (1 × 105 cells) at 2 wk compared with that of SK-Hep1 tumor (background). See quantification results in the text using region-of-interest analysis: a, SK-Hep1, 1 × 105 cells; b, SK-ND, 1 × 105 cells; c, SK-ND, 1 × 107 cells. T = thyroid; S = stomach; B = bladder.
DISCUSSION
The development of a variety of well-established radionuclide or optical reporter genes has been required to track grafted cells in vivo. Whereas human use of the reporter genes is warranted to follow up the fates and physiologic changes associated with proliferation or differentiation of grafted stem cells, these reporter genes should yield good sensitivity and not be immunogenic to human subjects (18). Another classical use of the reporter gene has been to monitor the therapeutic efficacy of a therapeutic gene delivered directly to the tissues or in a vehicle cell transduced with this therapeutic gene. To detect the spatial location and the level of expression in vivo, the therapeutic and reporter genes should be linked together, efficiently transfected, and stably maintained. Recently, HSV1-tk and hNIS have been widely examined as simultaneous therapeutic/reporter genes by themselves or in combination. More recently, D2R was proposed as a reporter gene that has various available radioligands for PET or possibly for SPECT (7,19–21). These radioligands pass the blood–brain barrier and bind specifically to the D2R transgene product, a membrane receptor reporter. 3-(2′-18F-Fluoroethyl)spiperone and 11C-raclopride are representative ligands for PET (22,23). The D2R transgene was successfully manipulated not to influence the intracellular physiologic activity of transfected target cells by mutation of specific base pairs (13,24), and this was proposed to be essential for the use of D2R as a reporter (7,25). This mutant D2R is believed to be expressed on the cellular membrane and bind dopamine analogs but does not transduce signals inside the cells (13). In this study, we chose hNIS and mutant D2R as dual reporter genes and constructed a membrane protein dual reporter system, which has a potential for future human use.
Many reports have supported the role of a dual reporter system as an experimental validation tool for molecular imaging using 2 reporter genes, such as GFP (green fluorescent protein) and hNIS (26) or HSV1-tk and D2R (19,25). These dual reporter systems have been used to evaluate the feasibility of in vivo use of a new reporter gene, and this feasibility was validated by comparison with the established reporters such as NIS or HSV1-tk (27). Many investigators prefer to use IRES binding of reporter transgenes to separate cotransfection of reporter vectors to guarantee simultaneous expression, but the downstream reporter transgenes showed variable attenuation in their expression and functional activities (19,28). Using a dual reporter system of D2R and HSV1-sr39tk, Yu et al. reported that the downstream reporter gene activity was even 0.1-fold compared with the gene placed upstream of the IRES (19). In addition to the usual position effect and copy number variability of transgenes, this downstream attenuation required the assessment of functional transgene activities for each cell line before use after transfection or selection. In this study, we found similar attenuation of the expression of mutant D2R placed downstream of the IRES in SK-ND cells compared with single D2R expression in SK-D cells (D2R expression directly regulated by the promoter).
Because we chose 2 membrane-bound reporter transgenes as a reporter system, a concern arose that there might be competition between these 2 exogenous transgenes in a cell. Also, because we selected clones showing highly enhanced functional activities of transgenes, the possibility of transgene competition between 2 membrane reporters was heightened. This competition phenomenon between 2 reporter transgenes was proven in our study, and we suspect that this competition was based on the fact that both of the reporters are membrane bound and highly overexpressed. When we transfected SK-N or SK-D cells further with luciferase (cytosolic protein) reporter vectors, the functional activities of hNIS and D2R were unchanged.
hNIS is well known for its many advantages as an ideal reporter gene, a well-understood biodistribution of its ligands (123I, 131I, to 99mTc-pertechnetate), and nonimmunogenic properties in humans. Even transgenic animals expressing hNIS transgene ectopically in cardiac myocytes did not show any problem in their cellular function and global/regional cardiac function as well as in survival (29). The presence of an iodine surplus inside the cardiomyocyte membrane did not appear to be harmful to the physiologic function in addition to its nonimmunogenicity. Ligands for this reporter gene vary from 123I, 131I, to 99mTc-pertechnetate as γ-tracers to 124I for PET. The real disadvantage of this reporter was that the injected radioisotopes accumulate in the thyroid, stomach, and the bladder and act as background to track the grafted cells. Furthermore, 124I or 99mTc-pertechnetate does not cross the blood–brain barrier and NIS-expressing cells cannot be tracked in the brain. D2R in our dual reporter system might have overcome this problem because the radioligands for D2R easily enter the brain. However, visibility of D2R reporter–carrying cells in the brain might also be compromised by endogenous striatal activities. How to suppress endogenous D2R activity or how to increase the sensitivity (and contrast) of D2R reporter activities are the next problems to solve for D2R reporter application for in vivo molecular imaging.
The biodistribution data for D2R expression using 3H-spiperone suggested that 3H-spiperone was efficiently accumulated in SK-ND tumors (Fig. 5B). The ratio of specific 3H-spiperone accumulation in transfected tumor to nontransfected tumor showed a plateau value of 2.2 until 24 h after 3H-spiperone injection. The tumor-to-blood ratio was more than desired; however, there was a high background of 3H-spiperone in the various internal organs and muscles. This is another limitation of D2R as a reporter in vivo.
The scintigraphic images of a nude mouse showed enhanced uptake of 99mTc-pertechnetate at the SK-ND tumor as the tumor mass increased. Tumor mass with inoculation of a large number of cells revealed hNIS activity 1 wk after the cell graft. When we injected the smaller number of SK-ND tumor cells, 99mTc-pertechnetate uptake increased proportionally as time passed. This occurred until the center of the tumor necrotized after 5 wk. We had confirmed that the dissected tumor (right hindlimb of the inoculation of 1 × 107 cells) had a huge necrotic area in the middle of the tumor at the time of sacrifice at 5 wk (data not shown). In hepatocellular carcinoma or glioma models, the linear relationship of the reporter transgene activities, regardless of hNIS or D2R, and cellular numbers was sure to be maintained both in vitro and in vivo.
CONCLUSION
The benefits of using this dual reporter system of hNIS and D2R might be summarized as follows. The complementary radiolabeled probes are readily available for each of these reporter genes. If hNIS is used as a therapeutic gene using 131I or 188Re, D2R might be used as a PET reporter gene. However, when we use this dual reporter system to follow the therapeutic response—considering that 131I or 188Re therapy itself might disturb the correlation between hNIS and D2R expression—the maintenance of this correlation should be confirmed again. As neither hNIS nor D2R is immunogenic in humans, this system has a potential for future human application. hNIS and D2R, if used for cell tracking, can complement each other for the identification of the spatial location of the grafted cells. D2R might be used to scrutinize the areas where the hNIS activity cannot be visualized or masked (near the thyroid or stomach and the brain). We successfully established an IRES-linked dual reporter system and characterized this system both in vitro and in vivo using hepatoma and glioma tumor cells. We also found that in a dual membrane-bound protein reporter system there was a competition between 2 reporter proteins as they were both highly expressed on the cellular membranes. Considering this competition and after in vitro confirmation of transgene activities in vitro, we expect that our dual reporter system might be used for tracking the grafted cells' homing or the quantification of the cell mass of grafted tumors by measuring functional activities of in vivo transgene expression. Further small-animal PET study is needed for verification of the imaging capability of the downstream D2R.
Acknowledgments
We thank Dr. Sanjiv Gambhir (Stanford University, Stanford, CA) for kind donation of his mutant D2R gene construct. This work was supported by the Nano Bio Regenomics Project of the Korean Science and Engineering Foundation.
Footnotes
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication September 17, 2006.
- Accepted for publication January 10, 2007.