Abstract
Multistep immune targeting holds great promise for radioimmunodiagnosis and therapy of cancer. Pretargeting of the tetrameric single-chain, variable-fragment streptavidin construct of the tetrameric monoclonal antibody CC49 with subsequent administration of radiolabeled 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA)-biotin has yielded promising results in TAG-72–expressing tumor xenograft models. A potential limitation of this approach, however, has been high and prolonged renal uptake of radioactivity. The objective of the current study, therefore, was to evaluate the reduction of kidney uptake of radiolabeled DOTA-biotin achieved by each of 4 different methods. Methods: A human pancreatic adenocarcinoma xenograft model (HPAC) in nude mice was used. The animals were intravenously injected with the antibody-streptavidin construct and a synthetic clearing agent (biotinylated N-acetyl-galactosamine) 24 and 4 h, respectively, before the administration of 67Ga-DOTA-biotin. For reduction of the renal uptake, different groups of mice were treated with streptavidin saturated with biotin, with several administrations of lysine or colchicine or with a succinylated antibody-streptavidin construct (resulting in a decreased electrical charge). All animals were sacrificed 24 h after injection of the 67Ga-DOTA-biotin for biodistribution and quantitative autoradiography (QAR) studies and selected animals underwent microSPECT/microCT studies. Results: There was marked targeting of the radiolabeled DOTA-biotin to tumor in all groups except in negative-control animals. Only succinylation of the scFv-CC49-streptavidin fusion protein significantly reduced (∼30%) kidney uptake without affecting tumor activity. QAR corroborated these results and demonstrated that radiolabeled DOTA-biotin localized selectively in the renal cortex. Among the other experimental groups, there was no change in kidney uptake of the radiolabeled biotin. Conclusion: In contrast to directly labeled antibodies and antibody fragments, administration of the negatively charged amino acid lysine was largely ineffective in pretargeting strategies with a single-chain-immuno-streptavidin fusion protein. Succinylation of the scFv-CC49-streptavidin construct, on the other hand, reduces kidney uptake of subsequently administered radiolabeled biotin, presumably by inhibiting reuptake of the fusion protein in the proximal renal tubules, and, therefore, could significantly reduce renal doses and improve therapeutic indices associated with multistep immune targeting approaches to radioimmunotherapy.
- multistep-targeting/pretargeting
- kidney dosimetry
- scFv-CC49-streptavidin-immune construct
- quantitative autoradiography
- microSPECT
Radiolabeled monoclonal antibodies (mAbs) and antibody fragments have shown promise for treatment of hematologic malignancies and, to lesser extent, solid tumors. In contrast to hematopoietic malignancies, radioimmunotherapy of solid tumors has been largely ineffective because of suboptimal tumor uptake, slow elimination of activity from blood, and marrow toxicity. In pretargeted radioimmunotherapy approaches, a nonradioactive mAb-construct is first administered, allowing for maximal tumor uptake over time without irradiation of blood and marrow, followed by administration of the radionuclide in the form of a small molecule that binds rapidly to the tumor-localized mAb-construct with high affinity and specificity. Thus, pretargeting can result in high tumor-to-tissue ratios and rapid blood clearance of the small radioligand (1,2).
Among the different pretargeting approaches are (strept)avidin/biotin, such as mAb/hapten (bispecific mAb), and oligonucleotide/antisense oligonucleotide analogs, with or without a “clearing step” before administration of the radiolabeled compound to bind to and accelerate the clearance of circulating mAb-constructs out of the blood (1,2). The (strept)avidin/biotin system is often used because of the very high binding affinity (∼1013–15·M−1) between (strept)avidin and biotin (1,3,4). An adaptation of this approach is the pretargeting method, consisting of the following 3 steps: first, the mAb-streptavidin construct is administered and allowed to circulate and accumulate in the tumor; second, the nontumor-bound mAb-streptavidin construct is removed from the circulation by administration of a synthetic clearing agent; third, the radiolabeled biotin is administered for targeting and irradiation of the tumor (5–10). Because of its small size, the egress of the biotin from the circulation is rapid, allowing either binding to the antibody-streptavidin conjugate already localized in the tumor or clearance through the kidneys. This approach is already being evaluated clinically (11–13).
In the current study, we used the single-chain, variable fragments (scFv) of the murine monoclonal antibody CC49 linked to streptavidin (8,13). The antibody fragment targets the tumor-associated glycoprotein-72 (TAG-72) antigen widely expressed on adenocarcinomas. This extracellular antigen is not internalized and is, therefore, well suited to the pretargeting approach. The clearing agent was a biotinylated poly-N-acetyl-galactosamine compound that complexes with the circulating streptavidin construct and is specifically extracted from the circulation by the liver. The biotin was labeled with a radiometal via the 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) chelate.
Several recent studies have described renal radiotoxicity using this otherwise promising pretargeting approach (12,14,15). Streptavidin itself has high and sustained uptake in the kidney. Biodistribution studies conducted with radioiodinated streptavidin have shown a high renal concentration (25–35 %ID/g [percentage injected dose per gram]) by 4 h after injection and persisting through 72 h after injection and even later (9,14), with no other appreciable tissue localization (16). Although variable in magnitude (16–18), high kidney uptake of streptavidin and streptavidinated proteins occurs generally. The exact mechanism of renal uptake remains unclear, however.
This article addresses different approaches to reducing the high kidney uptake and associated, potentially dose-limiting renal toxicity of mAb-streptavidin constructs. We evaluated 4 different methods: (i) blocking with high doses of streptavidin saturated with biotin, (ii) blocking by coadministration of lysine, (iii) blocking the micropinocytosis in the nephron cells by administration of colchicine, and (iv) decreasing the electrical charge (pI) of the mAb-streptavidin construct through chemical modification.
MATERIALS AND METHODS
Materials
The radionucleotide 67GaCl3 (half-life [t½] = 3.26 d) in 0.1N HCl was obtained from MDS Nordion. 68Ga-HCl (t½ = 67.6 min) was obtained by elution of a 68Ge/68Ga generator, extracted, and back-extracted as 68GaCl3 as described elsewhere (19). The targeting agent used in this study, a genetically engineered fusion protein of the single-chain, variable regions (scFv) of the murine mAb CC49 linked to the genomic full-length streptavidin, was provided by NeoRx Corp. and produced as described elsewhere (10,20,21). The fusion protein spontaneously assembles itself into a proteolysis-stable, tetrameric structure (molecular weight ∼ 176,000) and has been shown to be immunoreactive with bovine submaxillary mucin and the human colon carcinoma cell line LS174T and to bind biotin at an average occupancy of 3.7 of the 4 possible sites (10,21). The synthetic clearing agent consists of a bifunctional moiety with multiple N-acetyl-galactosamine residues (GalNAc)16 linked to biotin (molecular weight ∼ 8,652) (7,21). It binds rapidly to the circulating antibody-streptavidin construct, and the resulting complex clears rapidly from the circulation into the liver by the asialogalactose receptor present on hepatocytes (5,21,22). Biotin-LC-NM-(GalNAc)16 and DOTA-biotin (5) were also provided by NeoRx Corp.
For infusion, l-lysine ethyl ester dihydrochloride (62880; Fluka/Sigma-Aldrich) and d-lysine monohydrochloride (L5876; Sigma Chemical Co.) were dissolved in phosphate-buffered saline (PBS; 0.1 mol/L phosphate buffer, 0.0027 mol/L potassium chloride, 0.137 mol/L sodium chloride) and titrated with 1N NaOH to pH 7.4 (final concentrations, 40 mg/mL and 200 mg/mL, respectively). Colchicine (C-9754; Sigma Chemical Co.) was prepared for infusion by dissolving it in PBS to a final concentration of 250 μg/mL.
Radiolabeling of DOTA-Biotin
Ten microliters (10 μg in 0.3 mol/L ammonium acetate) of the biotin-linked chelator DOTA were added to 74 MBq (2 mCi) 67GaCl3 dissolved in 0.3 mol/L ammonium acetate, pH 5.0 (∼50 μL) and boiled for 5 min. Five millimole per liter DTPA was added to bind any free radiometal. The radiochemical purity, >99%, was determined by gradient high-performance liquid chromatography or by binding to avidin-coated agarose beads (Sigma Chemical Co.). DOTA-biotin was labeled with 68GaCl3 using the same protocol.
Prebiotinylation of Streptavidin
Five milligrams streptavidin (∼15–17 U/mg, S0677; Sigma Chemical Co.) were added to 3 mL sterile PBS containing an excess of 150 μg d-biotin (EC no. 2003993; Fluka/Sigma-Aldrich) and incubated for 30 min at room temperature. For intraperitoneal administration, 300 μL of the resulting solution (500 μg prebiotinylated streptavidin) were injected per animal. In 3 mice, radiolabeled prebiotinylated streptavidin, prepared as follows, was injected. Streptavidin was first incubated with 68Ga-DOTA-biotin for 15 min to radiolabel the streptavidin and then added to d-biotin and incubated for another 15 min to block all remaining free biotin-binding sites.
Succinylation of scFv-CC49-Streptavidin Construct
The succinylation reaction was performed as previously described (23). A 160-μL aliquot of scFv-CC49-streptavidin (0.6 mg; 3.75 mg/mL) was added to 370 μL of 50 mmol/L NaHCO3 buffer (pH 8.5). To this solution 57 μg succinic anhydride (no. 14089; Fluka/Sigma-Aldrich) in 20 μL of dimethyl sulfoxide (DMSO) were added, incubated for 30 min at room temperature, transferred to a Centricon-10 tube, and concentrated to ∼200 μL. PBS (500 μL) was added and the solution was again concentrated; this wash step was repeated 5 times. The final concentration was determined to be 1.53 mg/mL and the protein recovery was >97%. To determine the isoelectric point of the succinylated compound, nondenaturing isoelectric focusing (IEF) was performed on a pH 3–10 gel (Bio-Rad Laboratories) with IEF protein standards (161-0310; Bio-Rad Laboratories). The gel was loaded with succinylated and nonsuccinylated fusion protein samples (∼25 μg) that were mixed 1:2 with sample buffer (50% glycerol) and run for 1 h at 100 V and another 2 h at 250 V under cooling conditions using IEF anode and cathode buffer (Bio-Rad Laboratories). Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences Corp.) using a wet system with 0.7% acetic acid in 2 h at 350 mA and then stained with Coomassie Blue R250 dye.
Biodistribution Studies
All animal experiments were approved by the Institutional Animal Care and Use Committee of Memorial Sloan-Kettering Cancer Center, and institutional guidelines for the proper and humane use of animals in research were followed. For all studies, animals were maintained on a biotin-deficient diet (Biotin Deficient Diet 5836; Purina Mills) for at least 5 d before radiopharmaceutical administration and until subsequent sacrifice to ensure stable serum biotin levels similar to those in humans (5). Biodistribution studies were conducted using outbred female athymic nude nu/nu mice bearing ∼5 mm3 HPAC human pancreas adenocarcinoma (CRL-2119; American Type Culture Collection) xenografts. Tissue culture–maintained HPAC cell solutions (2–5 × 106 cells in 50 μL) were mixed 1:1 with Matrigel (BD BioSciences) and implanted subcutaneously in the shoulder or back region 7–10 d before initiation of the study. Usually there were 5 mice per experimental group. The mice were injected via the tail vein with 600 μg (3.488 nmol) of scFv-CC49-streptavidin fusion protein in 150 μL of normal saline (time [t] = −24 h), 100 μg (11.558 nmol) of clearing agent biotin-(GalNAc)16 in 100 μL normal saline (t = −4 h) and, finally (t = 0) 1 μg (1.24 nmol) of 67Ga-DOTA-biotin (∼6.66 MBq [∼180 μCi]) in 100 μL of normal saline (5,10). All mice were sacrificed 24 h after injection of the radiotracer. Blood, selected normal organs, and tumors were removed, and tissues and organs were blotted, weighed, and counted in an automatic scintillation well-type γ-counter (LKB Wallac 1282 Compugamma CS; Pharmacia ) with standards of the injected dose. Net counting rates were corrected for physical decay to the time of injection and expressed as the percentage of injected dose per gram of the tissue (%ID/g).
In the streptavidin-blocking studies, 4 groups of athymic mice were injected with 500 μg per mouse of the biotinylated streptavidin 1, 4, 24, and 48 h before administration of the scFv-CC49-streptavidin construct. Two additional groups received streptavidin at the 4- and 24-h respective times but no immune construct, and a final group received neither streptavidin nor the immune construct. For the lysine-blocking studies, each mouse received 4 intraperitoneal injections of either 10 mg l-lysine ethyl ester (4 × 0.4 mg/g) or 50 mg d-lysine (4 × 2 mg/g) in 250 μL sterile PBS 10 min before and 1, 2, and 3 h after injection of the streptavidin construct. In the colchicine pretreatment studies, each mouse received an intraperitoneal injection of 25 μg colchicine (1 μg/g) in 100 μL sterile PBS 5 h before administration of the streptavidin construct. In studies with succinylated scFv-CC49-streptavidin, mice received the succinylated construct instead of the unmodified fusion protein.
Quantitative Autoradiography (QAR)
From all animals sacrificed for biodistribution studies, the contralateral kidney and part of the tumor were sectioned (10 μm) in a cryostatic microtome (HM 500 M; Microm GmbH) after snap-freezing in liquid isopentane (−80°C) and cryofixation (OCT Tissue-Tek; SAKURA Finetek U.S.A. Inc.). After air drying, the slide-mounted sections were covered with a sheet of 5-μm-thick mylar and pressed against a phosphor screen (type BI) in a light-tight cassette and the screen was exposed for 1–3 d. The screen was then scanned in a Molecular Imager System (model GS-363; Bio-Rad Laboratories) using a laser scanner at a spatial resolution of 100 μm. Standards were prepared with 1M filter papers (Whatman Inc.) saturated with serial dilutions of a stock solution of 67GaCl3 and exposed simultaneously with the tissue sections. For tissue activity quantitation, regions of interest (ROIs) were manually drawn circumscribing the whole kidney and, separately, the cortex and the medulla, and the %ID/g was calculated based on the standard counting rates.
Imaging
In 3 mice, a PET scan was performed using a dedicated 3-dimensional small-animal PET scanner (model R4; CTI Concorde Microsystems, LLC) 1 h after tail vein injection of prebiotinylated streptavidin labeled with 68Ga-DOTA-biotin and d-biotin. Mice were anesthetized using a vaporizer (VetEquip) at a flow rate of 1 L/min O2 and 1%–1.5% isoflurane (Forane; Baxter). Animals were placed prone on the imaging palette and data (typically ∼10 million coincidence events) were acquired for 15 min using an energy window of 250–750 keV and a coincidence timing window of 6 ns. The resulting list-mode data were sorted into 2-dimensional histograms by Fourier rebinning and transverse images reconstructed by filtered backprojection into a 128 × 128 × 64 (0.82 × 0.82 × 1.2 mm) matrix. No attenuation, scatter, or partial-volume averaging correction was applied.
microCT and microSPECT were performed on the X-SPECT system (γ-Medica, Inc.). Mice were scanned 23 h after injection of 67Ga-DOTA-biotin using the anesthesia protocol described above; both scans were performed consecutively, without moving the animal, to allow precise registration of the microCT and microSPECT images. The microCT imaging parameters were as follows: x-ray tube voltage, 50 kVp; filament current, 0.6 mA; 256 projection views; cone-beam reconstruction. microSPECT imaging parameters were as follows: energy windows, 159–195 and 222–271 keV (summed); parallel-hole, medium-energy collimators; 64 projection views, at 90 s per view. SPECT data were reconstructed using a filtered backprojection algorithm and with postfiltering using a Butterworth low-pass filter with a cutoff frequency of 0.5 cm−1 and an order of 4. Again, no attenuation, scatter, or partial-volume averaging correction was applied.
Statistical Analysis
To compare the differences in biodistribution among the experimental groups, the Student t test was performed. Differences at the 95% confidence level (P < 0.05) were considered significant.
RESULTS
Blocking of Kidney Uptake by Pretreatment with Prebiotinylated Streptavidin
Figure 1A shows a representative maximum-intensity-projection image of a microPET study of an HPAC xenograft-bearing mouse administered 68Ga-labeled prebiotinylated streptavidin. It demonstrates that the tracer is mostly in the circulation (i.e., heart and large vessels) and in the kidneys. The urinary bladder has relatively little activity, however, consistent with stable labeling of the high-molecular-weight scFv-streptavidin complex.
(A) microPET maximum-intensity-projection image (coronal view) of the in vivo distribution of 68Ga-DOTA-biotin-streptavidin at 90 min after injection. High blood-pool and renal activities are prominently seen. (B) Quantitative autoradiograms of 67Ga-DOTA-biotin 24 h after injection following pretargeting with scFv-CC49-streptavidin and previous treatment with prebiotinylated streptavidin: kidney and tumor phosphor-plate autoradiograms. Kidneys, HPAC tumor xenografts, and standards (1 μL each of 0.33 kBq [0.0088 μCi] × 1, × 1/10, and × 1/100, respectively) are shown in the top, middle, and bottom rows, respectively. Four groups received prebiotinylated streptavidin 1, 4, 24, and 48 h before administration of the pretargeting protocol with the scFv-CC49-streptavidin construct, respectively, the clearing agent, and 67Ga-DOTA-biotin; the control group received no streptavidin.
Animals in all 5 experimental groups received the CC49-antibody-streptavidin construct 24 h and the clearing agent 4 h before the injection of 67Ga-DOTA-biotin, 4 of these groups were pretreated with prebiotinylated streptavidin at respective time points. Two additional groups also received the prebiotinylated streptavidin at 4 and 24 h but without receiving the immune construct. Another group (control) received neither the streptavidin nor the immune construct. All animals were sacrificed 24 h after the injection of 67Ga-DOTA-biotin. The biodistribution results are shown in Table 1 and representative autoradiograms of the contralateral kidneys and parts of the tumors are shown in Figure 1B.
Distribution of 67Ga-DOTA-Biotin 24 Hours After Injection Following Pretreatment with Prebiotinylated Streptavidin at Different Time Points and Pretargeting with scFv-CC49-Streptavidin
Kidney uptake was significantly lower if no scFv-CC49-streptavidin construct was administered, as indicated by the kidney activity concentrations of both control groups (P = 0.0005). The renal activity depends largely on administration of the streptavidin immune construct, with only a minor portion of the renal activity accounted for by the radiolabeled DOTA-biotin itself. Depending on the time of administration of the prebiotinylated streptavidin, the increase in renal activity was quite variable. Kidney activity was principally in the renal cortex, as demonstrated by the autoradiograms. The biodistribution data demonstrate, however, that only the activity in the kidneys, but not in other organs, was affected by pretreatment with streptavidin. The spatial distribution of activity within the tumor xenografts was markedly inhomogeneous, as seen in the tumor autoradiograms. Interestingly, the tumor uptake was significantly influenced by administration of the streptavidin, as seen by comparison of the control group and the 4-h-group tumor activity concentrations; neither of these groups received the immune construct, but 1 was treated with prebiotinylated streptavidin and showed a higher activity concentration in the tumors (P = 0.017).
Lysine Blocking of Kidney Uptake
This experiment was conducted to assess blocking by lysine administration of kidney uptake of radiolabeled DOTA-biotin after pretargeting with scFv-CC49-streptavidin. The mice received 4 hourly intraperitoneal injections of 10 mg l-lysine ethyl ester. At 24 h after injection of 67Ga-DOTA-biotin, there was no significant difference in biodistribution between the experimental apparent found at this time point (Table 2).
Distribution of 67Ga-DOTA-Biotin 24 Hours After Injection Following Pretargeting with scFv-CC49-Streptavidin and Lysine Treatment
Blocking of Kidney Uptake by Inhibition of Tubular Micropinocytosis with Colchicine
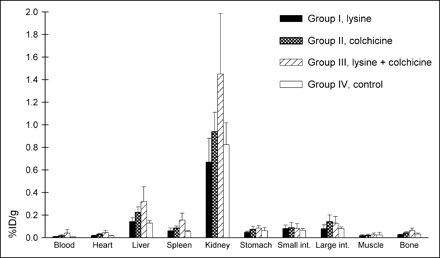
This study was conducted to assess the effect of colchicine on the inhibition of renal uptake of the streptavidin construct. Furthermore, blocking of the kidney uptake with higher-dose d-lysine and a possible synergistic effect of blocking of kidney uptake with colchicine and high-dose lysine were investigated. The comparative biodistributions of the radiolabeled DOTA-biotin at 24 h after injection are presented in Figure 2. High-dose lysine alone or in combination with colchicine did not affect the activity in the kidneys or any other organ.
Distribution of 67Ga-DOTA-biotin 24 h after injection following pretargeting with scFv-CC49-streptavidin and treatment with d-lysine or colchicine in athymic mice. Columns represent mean %ID/g and error bars represent SD. Four groups of animals were used; average animal weight was 25.7 ± 0.41 g. Groups I and III received intraperitoneal d-lysine (4 × 50 mg) 10 min before and 1, 2, and 3 h after administration of the antibody-streptavidin construct. Five hours before administration of the construct, groups II and III were treated with 25 μg intraperitoneal colchicine. All animals received 600 μg of antibody-streptavidin construct and 100 μg of clearing agent 24 and 4 h, respectively, before injection of 67Ga-DOTA-biotin (10.18 ± 0.22 MBq [275 ± 6 μCi]) and were sacrificed 24 h later. Statistical analysis comparing uptake of the groups showed no significant differences. int. = intestine.
Decreasing Kidney Concentration with Succinylated Streptavidin Construct
The succinylated, and charge-altered, streptavidin-immune construct (23) was evaluated for reducing the kidney uptake of subsequently administered radiolabeled DOTA-biotin. The results are shown in Table 3. A significant decrease in the kidney activity in the animals that received the succinylated construct was noted in comparison with that in the control group (P = 0.009), whereas the activity concentration remained unchanged in the other organs and in tumor. The mice pretargeted with the succinylated construct had 31% lower kidney activity than animals receiving the unmodified construct. Most important, the tumor uptake was not affected by the use of the succinylated antibody-streptavidin construct.
Distribution of 67Ga-DOTA-Biotin 24 Hours After Injection Following Pretargeting with Succinylated scFv-CC49-Streptavidin or Unmodified Construct
In Vivo Biodistribution and Imaging
Immediately before sacrifice, animals that underwent the standard pretargeting protocol with scFv-CC49-streptavidin or with the succinylated construct and subsequently received the clearing agent and 67Ga-DOTA-biotin, were imaged with our X-SPECT microCT/microSPECT. Figure 3 shows a representative image of a mouse pretargeted with the succinylated construct. Consistent with the biodistribution data, there was clear visualization of the subcutaneous tumor xenograft on the right shoulder of the mouse. There was renal activity (comparable to body background activity). The overlay of the CT and SPECT images shows the superimposition of the anatomy and radionuclide distribution.
microSPECT images of 67Ga-DOTA-biotin after pretargeting with succinylated scFV-CC49-streptavidin construct (middle row), microCT images (top row), and overlays of registered microSPECT and microCT images (bottom row) in transverse, coronal, and sagittal projections (from left). Scans were performed ∼23 h after injection of radiolabeled DOTA-biotin and immediately before sacrifice and without moving the animal between the microSPECT and microCT scans.
QAR
In all animals, parts of the tumor and the contralateral kidney of the mice were used for QAR. Kidney and tumor autoradiograms were consistent with the biodistribution data, with their relative intensities reflecting the relative activity concentration (Figs. 1B and 4A). The quantitative autoradiograms were also used for calculation of the tracer uptake, corroborating the biodistribution data (Table 3; Fig. 4B). Importantly, the autoradiograms showed a much higher concentration of activity in the cortex than that in the medulla in animals receiving either the succinylated or the unmodified streptavidin immune construct (Fig. 4A). However, the autoradiograms showed that both the whole-kidney and the renal cortex activities were substantially reduced (by 39%) with the succinylated construct—without any significant effect on tumor activity (Figs. 4A and 4B).
(A) Quantitative phosphor-plate autoradiograms 24 h after injection of 67Ga-DOTA-biotin following pretargeting with scFv-CC49-streptavidin or with the succinylated construct. Typical kidney and tumor sections from 2 representative mice in each of the 2 foregoing groups are shown. (B) Activity concentrations (%ID/g) in whole kidney, cortex, and tumor derived from conventional biodistribution measurements (left) and from quantitative autoradiograms (right). Columns represent mean %ID/g and error bars represent SD. Animals received 7.47 ± 0.17 MBq (202 ± 4.5 μCi) 67Ga-DOTA-biotin and were sacrificed 24 h later (average animal weight = 26.4 ± 0.88 g; average tumor weight = 63 ± 9 mg).
DISCUSSION
Streptavidin is an important reagent for many applications, including, in particular, multistep radioimmunotherapy, because of its high affinity for biotin. However, the streptavidin-based pretargeting approaches have 2 potential disadvantages: streptavidin is highly immunogenic, which may limit the ability to administer multiple cycles of therapy, and relatively high radiation doses to the kidneys have been reported in some trials using radiolabeled streptavidin. Decreasing the uptake of streptavidin in the kidney would reduce the renal radiotoxicity of pretargeting therapy approaches (16,17).
In the current study, different methods for reducing the renal uptake of a streptavidinated immune construct were evaluated. Such constructs—whether chemically synthesized or recombinant—have been used for a variety of different mAbs (5,6,9–12,24,25). We used a genetic fusion protein of streptavidin and the scFv of the mAb CC49 that targets TAG-72, an extracellular glycoprotein expressed by several adenocarcinomas (26). CC49 does not exhibit significant binding to any normal human tissue. Therefore, this construct should be very useful in pretargeting radioimmunotherapy. Our pretargeting approach has been previously described by investigators from NeoRx Corp. The tumor is first targeted by a nonradioactive antibody-streptavidin conjugate. The conjugate is then eliminated from the circulation by complexation with a clearing agent, consisting of multiple N-acetyl-galactosamine biotinylated residues, and catabolism of the complex in the liver through the interaction of the galactose groups and hepatic asialoglycoprotein receptors (22). In the final step, radiolabeled DOTA-biotin, a small molecule, is administered and rapidly either binds to sites on the tumor-localized streptavidin construct or is excreted via the kidneys. The particular scFv-CC49-antibody-streptavidin construct used in the current study is already undergoing clinical evaluation (13). As in our studies, however, these clinical trials have shown relatively high and persistent renal activities (27). Dose-dependent renal radiotoxicity from systemically administered radionuclides (e.g., in the form of radiolabeled DOTA-biotin) is difficult to predict on the basis of acute, high-rate external-beam radiation therapy experience because of marked differences between external and internal irradiations in terms of both the temporal and the intrarenal spatial distributions of radiation dose. Data for nephrotoxicity of radiometal-labeled immune conjugates (28) demonstrate that renal toxicity occurs at renal doses severalfold lower than those predicted based on external-beam toxicity data (14). It would appear, therefore, that the kidney—not the bone marrow—is the dose-limiting normal tissue for such pretargeting therapeutic strategies.
Renal activity concentrations of radiolabeled biotin administered after injection of streptavidin constructs does not change appreciably from 4 to 48 h after injection (16), and we therefore chose to evaluate biodistribution of biotin at 24 h after its injection. When only radiolabeled DOTA-biotin is administered (i.e., no streptavidin construct), the renal activity at 24 h is low (Table 1). When preceded by administration of the construct, however, high renal activities are achieved at 24 h and actually persist for several weeks (data not shown). In the current study, we detected much lower amounts of the streptavidin construct in the kidneys compared with the previously reported high (but variable) kidney uptakes of radiolabeled streptavidin (16–18). This difference may be related to the late time after injection, 48 h, at which we assayed renal activities of radiolabeled streptavidin or our method of labeling streptavidin.
Renal accumulation of streptavidin itself most likely proceeds in the same manner as that of other small proteins—that is, glomerular filtration followed by uptake in the proximal tubular cells (29). Although such tubular uptake is considered a high-capacity process, some proteins may interact with specific receptors in the peritubular region. The exact mechanism of the tubular reuptake of streptavidin (and conjugates) has not been established, however. Whether or not receptor bound, intrarenal streptavidin could be available for binding of circulating radiolabeled biotin (23). If streptavidin binding in the kidney is, in fact, receptor mediated and, therefore, saturable, administration of streptavidin that had all biotin-binding sites occupied (prebiotinylated) would preclude the binding of radiolabeled biotin molecules. It has been suggested that the renal accumulation of streptavidin is related to the “universal recognition sequence” RGD (amino acids Arg, Gly, Asp) present in adhesion proteins (e.g., fibronectin) that bind to cell-surface integrins (30,31). The RYD (Arg, Tyr, Asp) sequence found in streptavidin mimics the RGD receptor domain of fibronectin and could cause receptor-mediated binding to tubular cells (30). However, Wilbur et al. (23) was unsuccessful in attempting to saturate streptavidin receptor binding with prebiotinylated streptavidin. Therefore, it was suggested that the long retention time of streptavidin in the kidney is more likely due to its resistance to degradation of the proteolytically stable streptavidin in the lysozyme (31). However, only 15 μg of prebiotinylated streptavidin were administered in this study and it is possible that the relevant receptors were simply not saturated at this dosage (23).
Although renal concentrations of streptavidinated proteins characteristically are high, the concentrations reported vary greatly (16–18,32). In our study, we used high-dose (500 μg) prebiotinylated streptavidin for blocking a possible receptor-mediated renal uptake mechanism, varying the time of administration before the antibody-streptavidin construct. PET images of 68Ga-DOTA-biotin–labeled streptavidin showed little bladder activity, indicating that the 68Ga-DOTA-biotin was stably bound to the streptavidin. After pretreatment with large amounts of prebiotinylated streptavidin and pretargeting with the streptavidin construct, no apparent saturation of renal uptake was evident, regardless of the time of administration. These findings do not support a receptor-mediated uptake mechanism of streptavidin by renal tubular cells.
Another hypothetic mechanism for the observed high renal uptake of the streptavidin construct is normal tubular protein reabsorption, with binding between a free positive amino- or guanidino-group (33). This led to the discovery that lysine, or other positively charged amino acids, can block tubular absorption of small peptides. This approach is already being applied clinically without notable toxicity and has proven useful in decreasing renal accumulation of directly labeled antibody fragments and oligopeptides (34). Some studies have already shown that this technique also decreases the renal uptake of streptavidin by ∼30% (23). In the current study, high-dose d-lysine (or l-lysine ethyl ester) administered 4 times at hourly intervals demonstrably did not reduce renal uptake of our radiolabeled streptavidin construct.
Although the exact mechanism of the renal uptake of low-molecular-weight proteins and oligopeptides is not fully understood, the reabsorption process in the proximal tubule cell membrane occurs primarily via endocytosis of cell membrane. This may include specific or nonspecific electrostatic binding. The complex is then transported to the lysosomal apparatus for proteolysis, with recycling of the protein-binding molecule back to the cell surface (35). It is known that microtubule-dependent endocytosis plays a role in kidney uptake of oligopeptides in rats, for example. Colchicine prevents polymerization of microtubules in the cytoplasm and nucleus and, thus, inhibits the return of the cell-membrane components to the cell surface, resulting in decreased proximal tubule reabsorption of low-molecular-weight proteins and peptides (36). In rats, colchicine blocks tubule uptake of radiolabeled octreotide in a dose-dependent manner, with a maximal effect at a dose of 1 mg/kg (37). In the current study, we treated mice with colchicine alone or in combination with lysine and saw no reduction in kidney uptake of the streptavidin construct. This does not disprove a microcytosis-dependent uptake mechanism for the streptavidin construct, but it does indicate that treatment with colchicine will not reduce the kidney radiation burden in pretargeted therapy.
It is known that negatively charged macromolecules are restricted from crossing the glomerular wall (38) and this led to the hypothesis that modification of the overall charge of streptavidin might reduce renal uptake (39). Wilbur et al. (23) demonstrated that succinylation of the streptavidin molecule by modification of the lysine amines reduces its accumulation in mouse kidney and is superior to alternate methods, such as blockade with prebiotinylated streptavidin or administration of lysine, with kidney uptakes dependent on the level of succinylation (40), which is consistent with the hypothesis that tubular reabsorption depends on binding between positive groups on the protein and negative groups on the cell surface (33). Importantly, the succinylation of streptavidin does not appear to affect other important properties of streptavidin, such as biotin binding (40). In our study, we used the same modification technique and succinylated the entire scFv-CC49-streptavidin construct and made it anionic (pI ≈ 4.4). The resulting renal uptake of radiolabeled DOTA-biotin administered 24 h later was reduced by >30% in the whole kidney and by 39% in the renal cortex. Succinylation of the streptavidin construct thus resulted in a reduction of 44% in the calculated mean kidney absorbed dose for both 67Ga- and 90Y-DOTA-biotin. Activity concentrations in other normal organs and, importantly, in the HPAC tumor xenograft did not change. Our microSPECT studies with 67Ga-DOTA-biotin corroborated these findings.
In the current study, we used the human pancreatic carcinoma tumor HPAC out of several pancreatic models because its showed the highest expression of TAG-72, even though the tumors had only a moderate uptake compared with that in the LS174T human colon adenocarcinoma model typically used in studies of CC49 targeting (27). Thus, despite a heterogeneous distribution of activity within HPAC tumors (by phosphor-plate autoradiography) as well as a wide variability in uptake among the HPAC xenografts, a decrease in tumor uptake should be easily detectable. Importantly, the overall tumor uptake of the radiolabeled DOTA-biotin was not affected by any of the strategies designed to reduce renal uptake of activity. Although the tumor uptake of activity is probably insufficient for curative radioimmunotherapy of pancreatic cancer, pretargeting of the scFv-CC49-streptavidin fusion protein may prove useful for detection and imaging of pancreatic tumors.
CONCLUSION
Four methods of decreasing kidney uptake of radiolabeled DOTA-biotin in a pretargeted radioimmunotherapy strategy with the scFv-CC49-streptavidin construct and an intermediated clearing step were evaluated. Chemical modification of the construct by succinylation of lysine amines was the most effective in reducing kidney uptake and potential dose-limiting toxicity of radiolabeled biotin.
Acknowledgments
This study was supported, in part, by Department of Energy grant 95ER62039 and National Institutes of Health grants 1R2CA83084 and S10-RR017935. The authors wish to thank Donald Axworthy and Louis J. Theodore of Aletheon Pharmaceuticals, Inc., for valuable support and advice in the preparation of this manuscript. We thank Jose Campa for expert technical assistance.
References
- Received for publication April 28, 2005.
- Accepted for publication October 5, 2005.