Abstract
Detection of cholangiocarcinoma in extrahepatic bile duct strictures is a continuing challenge in clinical practice because brush cytology taken at endoscopic retrograde cholangiography has an average sensitivity of 50%. The aim of this study was to evaluate the effectiveness of dual-modality PET/CT using 18F-FDG for noninvasive differentiation of extrahepatic bile duct strictures. Methods: Twenty-two PET/CT studies were performed on 20 patients (10 women, 10 men; mean age ± SD, 63 ± 14 y) with extrahepatic bile duct strictures on endoscopic retrograde cholangiography. PET imaging was started 101 ± 22 min after injection of 369 ± 48 MBq of 18F-FDG. Blood glucose was 100 ± 20 mg/dL. PET images were reconstructed iteratively with attenuation correction based on a rescaling of the CT image. CT was performed within 1 min before the PET study, with the patient in the same position. CT was used to place a volume of interest 5 cm in diameter at the liver hilus for quantitative evaluation of PET images by means of standardized uptake values (SUVs). Results: Final diagnosis was histologically proven cholangiocarcinoma in 14 cases and benign causes of strictures in 8 cases without evidence of malignancy during a follow-up of 18 ± 3 mo. All patients with cholangiocarcinoma presented with focal increased uptake in the liver hilus with an SUV of 6.8 ± 3.3 (range, 3.9–15.8), compared with 2.9 ± 0.3 (range, 2.5–3.3) in patients with benign causes of strictures (P = 0.003). There was a clear cutoff SUV of 3.6 for detection of malignancy in the liver hilus. Conclusion: 18F-FDG PET/CT provided high accuracy for noninvasive detection of perihilar cholangiocarcinoma in extrahepatic bile duct strictures.
Klatskin’s tumor, or perihilar cholangiocarcinoma involving the bifurcation of the hepatic duct, accounts for approximately 70% of all bile duct cancers (1). More than 95% of all cancers of the biliary tree are adenocarcinomas (2). Cholangiocarcinoma is a well-known complication of primary sclerosing cholangitis (PSC). Nonetheless, differentiation of cholangiocarcinoma in extrahepatic bile duct strictures found on endoscopic retrograde cholangiography (ERC) or percutaneous transhepatic cholangiography is a continuing diagnostic challenge in clinical practice. The average prevalence of cholangiocarcinoma in PSC patients is about 10% (3). Tissue diagnosis by brush cytology taken at cholangiography is highly specific, but sensitivity has been reported to be between 18% and 70%, averaging 50% (4–6). Often, a combination of methods is recommended, including ERC with brush cytology, DNA analysis, and serum analysis of CA 19-9 and carcinoembryonic antigen, yielding an increased sensitivity of 70%–80% (4,7,8). MRI, CT, magnetic resonance cholangiopancreatography, and endoscopic ultrasonography might provide further valuable information; the exact extent of invasion, however, may be difficult to discern (3,9). Despite all the methodologic improvements in diagnostic imaging in the past decade, diagnosis of cholangiocarcinoma, especially in PSC, sometimes remains uncertain until invasive and aggressive approaches such as explorative laparotomy have provided a biopsy sample for histologic examination. Thus, identification of a sensitive and specific imaging modality would be a useful adjunct to existing procedures for detection of cholangiocarcinoma.
PET using 18F-FDG is a noninvasive imaging method for assessment of glucose metabolism in a variety of malignancies, including adenocarcinomas, and CT is the most frequently used cross-sectional imaging technique for morphologic tumor detection (10). The combination of both functional and morphologic cross-sectional imaging has been considered a new road map for imaging studies in oncology, with CT adding the anatomic details that PET lacks (11).
The purpose of this study was to evaluate the effectiveness of combined PET/CT for noninvasive differentiation of extrahepatic bile duct strictures.
MATERIALS AND METHODS
Patients
Between December 2002 and June 2004, 22 whole-body 18F-FDG PET/CT studies were performed on 20 consecutive patients (10 women, 10 men; mean age ± SD, 63 ± 14 y) with extrahepatic bile duct strictures on ERC suggesting the presence of cholangiocarcinoma. The ERC findings included a lengthy stricture with an irregular margin and asymmetric narrowing. Extrahepatic bile duct strictures were classified according to Bismuth et al. (12). The median interval between ERC and 18F-FDG PET/CT was 6 d (range, 3–14 d). Seven PET/CT studies were performed on patients with cytologic findings of cholangiocarcinoma for primary staging before surgical or photodynamic therapy (PDT). Eight PET/CT studies were performed on patients with PSC or other benign causes of strictures and with negative cytology findings in order to exclude cholangiocarcinoma and avoid surgery in cases of negative PET findings. Seven studies were performed on patients for therapy control after PDT of cholangiocarcinoma. PSC was diagnosed according to typical cholangiographic findings of multiple strictures and dilatations of the intra- or extrahepatic bile ducts and other published diagnostic criteria (13). All patients provided written consent after the nature of the imaging study had been fully explained.
Imaging Studies
All imaging studies were performed on a PET/CT scanner (Biograph; Siemens Medical Solutions, Inc.) with a 15.8-cm axial field of view and an in-plane spatial resolution of 4.6 mm. PET imaging was started 101 ± 22 min after intravenous injection of 369 ± 48 MBq of 18F-FDG through an anterior cubital vein. Blood glucose measured before injection was 100 ± 20 mg/dL. The acquisition time was 5 min per bed position, with 5–7 bed positions covering thorax, abdomen, and pelvis. CT was performed within 1 min before PET with the patient in the same position. The acquisition parameters for dual-detector helical CT were 130 kV, 40 mAs, 0.8 s per CT rotation, 5-mm slice thickness, and a pitch of 1.5. One liter of an oral contrast agent was ingested within 1 h before CT for better delineation of adjacent intestinal structures. Attenuation correction for PET was based on a rescaling of the CT image as described elsewhere (14). No intravenous contrast agent was given, to avoid possible reconstruction artifacts on PET images of the liver (15). CT was used to define a perihilar volume of interest (VOI) 5 cm in diameter for quantitative evaluation of PET images. Maximum standardized uptake value (SUVmax = activity concentration/injected dose/body weight) was obtained for the perihilar VOI of each patient. Because SUVmax might represent the peak activity of only a single voxel, a 75% isocontour area within the perihilar VOI was defined to determine the 75% SUV (SUV75%). Mean SUVmax and SUV75% were compared between patients with and without Klatskin’s tumor. Quantitative evaluation of PET images was performed using e.soft 3.0 software (Siemens Medical Solutions, Inc.).
Statistical Analysis
Mean values and SDs, as well as 95% confidence interval of the mean of SUVmax and SUV75%, are given for patients with perihilar cholangiocarcinoma and with benign causes of strictures. The comparison of mean SUVmax and SUV75% between patients with and without Klatskin’s tumor was performed by means of 1-way ANOVA and displayed as box plots. All statistical evaluation was done using SPSS 12.0 software (SPSS Inc.) for Windows (Microsoft).
RESULTS
18F-FDG PET Findings and Clinical Course of Disease
Perihilar adenocarcinoma of the bile ducts was confirmed histologically in all patients who underwent 18F-FDG PET/CT before surgical or PDT treatment. All these patients had focal increased 18F-FDG uptake in the liver hilus. The tumors included 3 Bismuth IV and 2 each Bismuth II and III tumors, with 5 being American Joint Committee on Cancer (AJCC) stage II and 2 being AJCC stage III.
Seven of 8 patients with suspected benign causes of strictures had no evidence of malignancy during further follow-up of 18 ± 3 mo (range, 12–21 mo) and had no perihilar increased metabolic activity. In the eighth patient, cholangiocarcinoma was suspected at ERC but several biopsy specimens taken at that time were negative. This patient (patient 12) had perihilar increased 18F-FDG uptake with an SUVmax of 4.2 and an SUV75% of 3.5 on 18F-FDG PET/CT. Therefore, she underwent partial resection of the liver 1–2 wk later, and cholangiocarcinoma was then confirmed histologically.
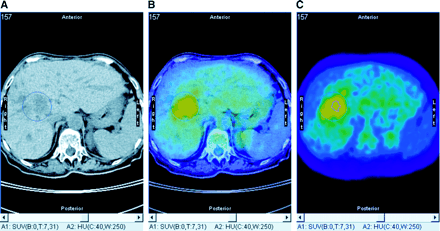
Seven PET/CT studies were performed on 6 patients for therapy control after PDT of Klatskin’s tumor; all tumors were Bismuth IV. Four patients had persisting perihilar hypodensity, and 2 patients showed tumor regression on CT. However, 5 of them had focal increased 18F-FDG uptake, including 1 of the 2 patients with decreasing tumor size on CT. All patients with focal increased 18F-FDG uptake experienced disease relapse within 5–14 mo, whereas the 1 patient lacking 18F-FDG uptake in the liver hilus had no evidence of remaining or recurrent malignancy at the end of 18 mo of follow-up. The 18F-FDG PET/CT study of this patient (patient 20) is shown in Figure 1. Figure 2 shows the 18F-FDG PET/CT study of patient 11, who had a partial response after PDT on CT but had increased 18F-FDG uptake in the remaining tumor and experienced relapse of disease 14 mo later.
18F-FDG PET/CT of patient with no evidence of malignancy after PDT. (A) Native CT image shows discrete hypodense areas in liver segments 4B and 5. (B) Fused image (CT, 50%; PET, 50%) enables anatomic correlation of glucose metabolism. (C) 18F-FDG PET image shows 75% and 100% isocontour areas used for SUV analysis (SUVmax = 3.2 and SUV75% = 2.5). Note atrophy of left liver lobe.
18F-FDG PET/CT of patient with residual Klatskin’s tumor after PDT and Yamakawa stent implantation. (A) Native CT image shows large hypodense area in liver hilus. (B) Fused image (CT, 50%; PET, 50%) enables anatomic correlation of increased glucose metabolism. (C) 18F-FDG PET image shows 75% and 100% isocontour areas used for SUV analysis (SUVmax, 5.0, and SUV75%, 4.1).
Further details on patients, including indications for 18F-FDG PET/CT studies, results of quantitative analysis, histology, and outcome, are shown in Table 1.
Characteristics, 18F-FDG PET/CT Indications, Perihilar SUV, Histology, and Outcome of 20 Patients with Extrahepatic Bile Duct Strictures
Quantitative Evaluation of 18F-FDG PET/CT
The perihilar SUVmax of patients without cholangiocarcinoma was 2.87 ± 0.25, with minimum and maximum values of 2.47 and 3.24 and a 95% confidence interval of 2.66–3.08. The perihilar SUVmax of patients with Klatskin’s tumor was 6.81 ± 3.3, with minimum and maximum values of 3.85 and 15.8 and a 95% confidence interval of 4.89–8.72. The geometric and arithmetic means between the highest SUVmax of patients without cholangiocarcinoma and the lowest SUVmax of patients with cholangiocarcinoma were 3.54 and 3.55, respectively. Thus, an SUVmax of 3.6 was defined as the cutoff for detection of malignancy in central bile duct strictures. Using the isocontour SUV75% in the anatomically defined perihilar VOI, the SUV75% of patients without cholangiocarcinoma was 2.32 ± 0.19, with minimum and maximum values of 2.08 and 2.66 and a 95% confidence interval of 2.17–2.48. The perihilar SUV75% of patients with Klatskin’s tumor was 5.61 ± 2.69, with minimum and maximum values of 3.13 and 12.8 and a 95% confidence interval of 4.06–7.17. The geometric and arithmetic means between the highest SUV75% of patients without cholangiocarcinoma and the lowest SUV75% of patients with cholangiocarcinoma were 2.885 and 2.895, respectively. Thus, an SUV75% of 2.9 was defined as the cutoff for detection of malignancy in central bile duct strictures.
The differences in SUVmax and SUV75% between patients with and without cholangiocarcinoma were significant: P = 0.003 for SUVmax and P = 0.003 for SUV75%. The distribution of SUVmax in patients with and without Klatskin’s tumor is shown in Figure 3, and that of SUV75% is shown in Figure 4.
Box plot analysis of perihilar SUVmax in 22 patient studies with and without cholangiocarcinoma. ○ = outliers; * = extreme cases; CC = cholangiocarcinoma.
Box plot analysis of perihilar SUV75% in 22 patient studies with and without cholangiocarcinoma. ○ = outliers; * = extreme cases; CC = cholangiocarcinoma.
DISCUSSION
The usefulness of 18F-FDG PET for detection of cholangiocarcinoma has been shown in just a few studies, most of them including fewer than 20 patients (16–20). The first 2 studies did not specifically address cholangiocarcinoma but rather assessed malignant lesions of different origins in the liver, including hepatocellular carcinoma and liver metastases from colon carcinoma, in comparison with normal liver tissue (16,17). In 1992, Okazumi et al. performed dynamic 18F-FDG PET studies that included arterial blood sampling until 60 min after intravenous injection of the radiotracer (16). They observed a higher 18F-FDG uptake in the tumors than in the surrounding liver tissue at 60 min after injection and defined a k3-value (which describes the phosphorylative activity of hexokinase) of ≥0.025 as indicative of malignancy.
In 1998, Delbeke et al. studied 8 patients with cholangiocarcinoma (17). All had increased target-to-nontarget ratios (>2). SUVmax was 7.2 ± 3.2, with PET begun 68 ± 33 min after injection. The investigators concluded that conventional static 18F-FDG PET can distinguish malignant lesions in the liver. Also in 1998, Keiding et al. compared, for the first time, 18F-FDG liver uptake in patients with PSC but no cholangiocarcinoma, patients with PSC and cholangiocarcinoma, and patients with no liver disease (18). The investigators performed dynamic PET scanning of the liver for 90 min after injection of the radiotracer and included arterial blood sampling. Quantitative analysis was done by means of a VOI covering all voxels that enclosed at least 75% of the maximum radioactivity concentration. Using an estimate of the net metabolic clearance of 18F-FDG, K (in mL of plasma min−1 mL−1 of tissue), Keiding et al. could clearly differentiate all PSC patients with cholangiocarcinoma from those with PSC but without cholangiocarcinoma. However, the K-values of patients with PSC were significantly higher than those of patients without liver disease, suggesting increased glucose metabolism in PSC tissue. The investigators concluded that for detecting small and early malignant tumors in the liver, morphologic imaging procedures might be less suitable than 18F-FDG PET, because it revealed hot spots in areas that were not suspected on ultrasound and MRI in up to half the cholangiocarcinoma patients.
In 2001, Fritscher-Ravens et al. performed 18F-FDG PET, CT, and endoscopic retrograde cholangiopancreatography on 15 patients with PSC before surgical intervention (19). In 10 patients with adenocarcinoma of the bile ducts and tumor sizes between 0.9 and 4.5 cm, the tumors were clearly seen on 18F-FDG PET images 60 min after injection of the radiotracer, in contrast to 3 patients with mucinous adenocarcinoma. Two patients with benign Klatskin-mimicking chronic inflammatory lesions had false-positive PET findings, with target-to-nontarget ratios similar to those of cholangiocarcinoma. The authors suggested that 18F-FDG PET is more useful for the detection of distant metastases than for differentiation of malignant from benign lesions in the liver. In the same year, Kluge et al. performed 18F-FDG PET on 26 patients with adenocarcinomas of the bile ducts, 8 patients with benign lesions of the biliary tree, and 20 patients with no liver disease (20). Kluge et al. visually and quantitatively analyzed mean SUV in a 90% isocontour area around the most intense focus of uptake in the liver, with PET begun 50 min after injection of the radiotracer. In that study, visual analysis discriminated malignant from benign lesions significantly better than did SUV analysis, with an area under the receiver operating characteristic curve of 0.96 for visual analysis, compared with 0.87 for SUV analysis (P < 0.05). This difference disappeared after normalization of tumor SUV to that of normal liver tissue, which might be explained by higher 18F-FDG uptake in livers of PSC patients than in those of healthy controls, similarly observed in the study by Keiding et al. (18). A clear cutoff between malignant and benign lesions in the liver was shown in most of the quantitatively evaluated PET studies, that is, in the dynamic PET studies of Okazumi et al. (16) and Keiding et al. (18), in the SUV-based analysis of Delbeke et al. (17), and in our data.
The SUVmax cutoff for detection of cholangiocarcinoma was essentially the same in our study and the study of Delbeke et al. (17). In the Delbeke study it was >3.5, and in our study it was 3.6. The different uptake periods (101 ± 22 min vs. 68 ± 33 min) and acquisition times (5 min vs. 15 min per bed position) in our study and the Delbeke study would have led to the liver’s coming into the field of view at a similar time in both studies, about 110–120 min after injection. This would explain the similar cutoff of SUV for detection of cholangiocarcinoma in the liver in both studies, as the tumor uptake of 18F-FDG might change with time (21). However, the significantly reduced scanning time of 18F-FDG PET/CT increased patient comfort and enabled late acquisition without significantly interfering with departmental operations.
Several human and experimental studies found that a late imaging time for 18F-FDG PET better discriminated between tumor and inflammation (22–25). However, one cannot rule out the possibility that a few cases of focal granulomatous inflammation in the liver might give false-positive 18F-FDG PET findings, especially when PET is performed within 60–90 min after injection (19,20). In addition, false-negative 18F-FDG PET/CT results might be obtained when one is studying the rare subtype of mucinous carcinomas of the biliary tree, which seems not to show increased 18F-FDG uptake (19).
Despite our not using the full potential of the CT scan, since we did not administer intravenous contrast agents, we observed an improved anatomic localization of positive 18F-FDG PET findings rarely achievable with the standard 511-keV transmission images of a PET scanner (15). The direct incorporation of anatomic information derived from CT data into PET image reconstruction may be advantageous, since attenuation correction is essential for quantitative evaluation of PET images (26). Specifically, CT offers improved contrast and resolution and much lower statistical noise than do the standard 511-keV transmission images, especially for whole-body PET (15). In the present study, the anatomic information from the CT scan was used to define a VOI for quantitative evaluation of the liver hilus. By means of this advanced technique, 18F-FDG PET/CT differentiated 12 patients with cholangiocarcinoma from 8 patients without cholangiocarcinoma. These included 1 patient with repeatedly negative biopsy results, in whom 18F-FDG PET/CT was the first diagnostic procedure to give evidence of malignancy, which was then confirmed histologically a few weeks later when the patient underwent surgery. Furthermore, disease progression after PDT was correctly predicted with 18F-FDG PET/CT in all patients, including one for whom morphologic imaging procedures demonstrated tumor regression. In that patient, the combination of decrease in tumor size on CT and positive 18F-FDG PET findings suggested late relapse. And finally, 18F-FDG PET/CT correctly identified all patients with PSC and other benign causes of strictures in whom cholangiocarcinoma did not develop within the following 18 mo, including 1 patient without evidence of recurrent malignancy after PDT of cholangiocarcinoma.
CONCLUSION
18F-FDG PET/CT of the liver performed late—about 120 min after injection—might play a significant role in noninvasive detection and therapy control of Klatskin’s tumor.
Acknowledgments
We are grateful to Siemens AG Medical Solutions, Erlangen, Germany, whose financial support enabled the color reproduction of Figures 1 and 2 in this article.
Footnotes
Received Jan. 15, 2005; revision accepted Mar. 23, 2005.
For correspondence or reprints contact: Michael J. Reinhardt, MD, Department of Nuclear Medicine, University Hospital Bonn, Sigmund-Freud-Strasse 25, D-53105 Bonn, Germany.
E-mail: michael.reinhardt{at}ukb.uni-bonn.de