Abstract
This investigation examined the accuracy of dose calibrator activity measurement of the β-emitting radiopharmaceutical 90Y-ibritumomab tiuxetan. Methods: Five different facilities independently measured 90Y in a 10-mL syringe geometry with 30 dose calibrator models from 3 different manufacturers. The activities ranged from 81.4 MBq (2.2 mCi) to 1,406 MBq (38 mCi) over the volume range of 3–9 mL. Results: The mean dial settings for 90Y measurement were 375, 51 × 10, and 897 × 100 for Atomlab, CRC, and Mark V dose calibrators, respectively. The maximum volume dependence was 0.28%/mL. Conclusion: This study demonstrated that when measuring all volumes of 90Y-ibritumomab tiuxetan activity prescriptions, only a single dial setting for a given manufacturer’s dose calibrator is required for accurate measurements. Volume corrections are not necessary. For best accuracy, an individually determined dial value should be used.
The U.S. Food and Drug Administration has approved for commercial use the radioimmunotherapeutic agent 90Y-ibritumomab tiuxetan (90Y-Zevalin; IDEC Pharmaceuticals Corp.) for the treatment of non-Hodgkin’s lymphoma (1). This radiopharmaceutical is generally prepared at a commercial radiopharmacy and then supplied to medical facilities as a unit dosage in a 10-mL syringe with volumes ranging from 3 to 9 mL, dependent on the prescribed activity for an individual patient.
All Nuclear Regulatory Commission and Agreement State licensees must determine and record the activity of unsealed by-product material before medical use. Except in certain Agreement States, this activity determination does not require the use of a dose calibrator, pursuant to 10 CFR part 35.63 (2), provided unit dosages are obtained from an appropriately licensed manufacturer or preparer. However, because the package insert for 90Y-ibritumomab tiuxetan (1) states that patient dosages should be measured immediately before administration, licensees may prefer to directly measure activity with a dose calibrator.
Commercial reentrant ionization chambers (dose calibrators) are the de facto standard instrument to measure radioactivity in nuclear medicine. The dose calibrator measurement of β-emitting radionuclides depends on the bremsstrahlung radiation produced from the β-interaction with the source matrix, its container, and the calibrator chamber wall. The use of different volumes or containers may result in measurement errors, as is the case for low-energy photon emitters.
The purpose of this study was to involve the National Institute of Standards and Technology (NIST), dose calibrator manufacturers, and a commercial radiopharmacy in a common effort to investigate the applicability of a single calibrator dial setting for a particular manufacturer’s dose calibrator model and determine the significance of volume corrections for accurate measurement of the β-emitting radiopharmaceutical 90Y-ibritumomab tiuxetan in a syringe geometry.
MATERIALS AND METHODS
Dose calibrator measurements of 90Y were performed independently at 5 different sites: Capintec, Inc., NIST, Cardinal Health Nuclear Pharmacy Services, Cardinal Health Radiation Management Services (Nuclear Associates), and Sun Nuclear Corp. (sites 1, 2, 3, 4, and 5, respectively). Thirty dose calibrators of the pressurized argon well reentrant design were used, including CRC (Capintec), Mark V (Cardinal Health Radiation Management Services), and Atomlab (Sun Nuclear; distributed by Biodex Medical Systems Inc.). Table 1 summarizes the various dose calibrators and procedures used at each site.
Dose Calibrators Used and 90Y Activity Measurement Procedures at Each Site
The 90Y was delivered to site 3 by MDS Nordion in a 2-mL closed-septum vial containing a 90Y-chloride solution with a product data sheet that indicated the NIST-traceable activity concentration, volume, and total activity. The radioactive solution was transferred from the vial to a 10-mL syringe (Becton Dickinson & Co.). Dose calibrator measurements for 90Y after transfer to the syringe were based on vial measurements and an activity difference method. This procedure specifies measurement of the activity in the vial, both before and after removal of source material, but with the volume in the vial restored to its initial value with saline before remeasurement. The difference between these 2 vial measurements is the activity drawn into the syringe, which is still NIST traceable. Site 3 established traceability for the 90Y vial measurements through prior proficiency testing in a measurement assurance program with NIST. Calibrated dial settings for 90Y measurement for each calibrator were determined by adjusting the dial settings to read the correct activity; the standard uncertainty on the activity value in the present study was based primarily on the standard uncertainty on the activity provided by Nordion, which was ±5%. Sites 4 and 5 received a calibrated activity in a syringe from site 3; site 1 received a calibrated activity in an MDS Nordion vial and performed a nontraceable volumetric transfer of activity into the syringe.
Activity measurements were made either at a start volume of 9 mL and after sequential 1-mL volume withdrawals to a final volume of 3 mL or at a start volume of 3 mL and after sequential 1-mL volume additions to a final volume of 9 mL. The latter procedure was different in that only the volume was varied; the activity remained constant.
The correction factor for each volume was obtained by comparing the measured activity with the calculated activity for each volume. The volume correction factor is given by:
 where the calculated 90Y activity for each volume is a constant either for the 3-mL start volume or for the 9-mL start volume; it is the original calibrated activity multiplied by the respective volume divided by 9.
where the calculated 90Y activity for each volume is a constant either for the 3-mL start volume or for the 9-mL start volume; it is the original calibrated activity multiplied by the respective volume divided by 9.
The measurements performed by site 2 used a different approach involving a direct determination of the solution activity by liquid scintillation counting (3–5) and determination of calibration settings for a set of syringes, each independently prepared with different volumes covering the 3- to 9-mL range. The amount of 90Y solution added to each of the syringes was carefully controlled using an automated dispenser (Hamilton Co.) having an accuracy of 0.1%. In addition to the measurements made with 90Y-ibritumomab tiuxetan, the same procedure was repeated by site 2 for syringes containing 90Y in a carrier solution containing additional YCl3 and 1 mol·L−1 HCl to ascertain what effect the different source matrix may have on dose calibrator measurement because of differences in self-absorption or bremsstrahlung production.
The calibrated dial settings were also converted to response values for the ion chamber of the dose calibrator to compare the different manufacturers’ values. The relationship between chamber response, CR, normalized to 60Co and dial setting, DS, can be expressed as CR = 5.0/DS for Atomlab; CR = {(DS/1,083) + 0.0855}/M for CRC, where M is the display multiplier of 10; and CR = {120/(1,009 − DS)}/M for Mark V, where M is the display multiplier of 100.
RESULTS
The calibrated dial settings for 90Y measurement for each dose calibrator at each site are given in Table 2. For the Atomlab, the mean calibrated dial setting measured at site 5 was 375, with a range of 363–394 for 15 calibrators. Of the 15 calibrators, 10 were new and their dial settings exhibited a narrower range: 372–378. The mean calibrated dial setting at site 2 for 1 Atomlab was 393, with a range of 387–399.
Calibrated Dial Setting for Each Dose Calibrator at Each Site
For the 5 new CRC calibrators, the mean calibrated dial setting measured by site 1 was 50 × 10, with a range of 47 × 10 to 53 × 10. The mean calibrated dial setting at site 2 for 3 CRCs was 55.7 × 10, with a range of 54 × 10 to 58 × 10. The mean calibrated dial setting at site 3 for 3 CRCs was 47.3 × 10, with a range of 47 × 10 to 48 × 10. There was no overlap in the ranges between sites 1 and 2 or between sites 3 and 2. For the Mark V calibrators, the mean calibrated dial setting measured was 897 × 100, with a range of 896 × 100 to 897 × 100; no other sites studied these calibrators.
Based on the calibrated dial settings, the Atomlab chamber response value at site 2 was 0.01272 and the mean value at site 5 was 0.01333, a factor of 4.8% higher. The mean CRC response was 0.01369 at site 2 and 0.01317 at site 1, a factor of 3.8% lower. The mean CRC response at site 3 was 0.01292, a factor of 5.6% lower than the site 2 value. The mean Mark V response at site 4 was 0.01071.
For the measurements at site 2, the expanded (k = 2) uncertainty on the calibrated dial settings based on the liquid scintillation activity calibration was determined to be 1.6% and was calculated from the quadratic combination of the average SD and the mean deviate estimate calculated from range statistics (6). Similar uncertainty analysis was not performed at the other sites.
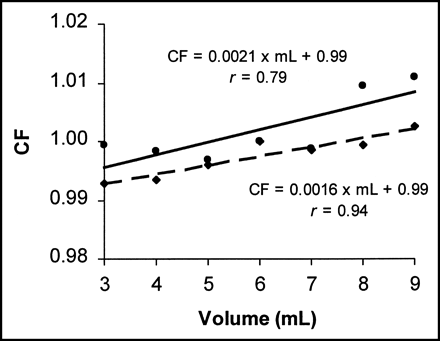
Volume correction factors determined by each site, normalized to 6 mL, are shown in Figures 1–4. The equation and correlation coefficient (r) for each line, resulting from linear regression analysis, are given in each figure. The largest volume dependence was determined to be 0.28%/mL. Using this maximum observed volume variation and the applicable volume range of 6 mL, the volume effect for all dose calibrators included in this study should be limited to 1.7%.
Correction factor (CF) as function of volume normalized to 6 mL, determined by site 1 based on measurements obtained on 1 CRC dose calibrator. Volume dependence is 0.28%/mL.
Correction factor (CF) as function of volume normalized to 6 mL, determined by site 2 based on measurements obtained on 3 CRC (•) and 1 Atomlab (♦) dose calibrators. Volume dependence is 0.21%/mL and 0.16%/mL, respectively.
Correction factor (CF) as function of volume normalized to 6 mL, determined by site 3 based on average of measurements obtained on 3 CRC dose calibrators. Bold line (♦) represents results for sequential 1-mL volume withdrawal from 9 to 3 mL, and dashed line (•) represents results for sequential 1-mL volume addition from 3 to 9 mL. Volume dependence is 0.20%/mL and 0.11%/mL, respectively.
Correction factor (CF) as function of volume normalized to 6 mL, determined by sites 4 (•) and 5 (▪) based on average of measurements obtained on 3 Mark V and 15 Atomlab dose calibrators, respectively. Volume dependence is 0.12%/mL and 0.13%/mL, respectively.
A comparison of 90Y measurements made with ibritumomab tiuxetan and the NIST standard solution in the identical measurement geometry indicated no difference in results to within the expanded measurement uncertainty of 1.6%. Moreover, variability in measurement results for both solutions due to variability in syringe manufacture was found to be less than 0.26% (SD), inclusive of 0.1% variability due to uncertainty in filling volume, at a volume of 5 mL for 8 syringes.
DISCUSSION
Proper dose calibrator measurement of pure β-emitting radionuclides is important for the safe and accurate dosing of various radionuclide therapies in nuclear medicine. According to NUREG-1556, volume 13 (7), and Nuclear Regulatory Commission Information Notice 2002-19 (8), accurate measurement of pure β-emitters is a potential problem. This study was performed to explore the pitfalls and examine the possible solutions when using dose calibrators for accurate measurement of 90Y-ibritumomab tiuxetan.
This study indicated that use of a single calibrated dial setting for a given manufacturer’s dose calibrator resulted in accurate measurements of 90Y in a syringe geometry regardless of volume in a 3- to 9-mL range. Although different model dose calibrators have very different dial settings, their chamber response values are very similar. The 90Y measurement variation based on the calibrator response values for the range of observed dial settings was determined to be within ±5%, which is the level of the standard uncertainty on the activity value as provided by the supplier, MDS Nordion. This uncertainty was propagated to the activity values provided to all participants in the study by the commercial radiopharmacy, with the exception of NIST, which performed its own independent activity calibration, and site 1, which used nominal techniques.
For best accuracy, it is recommended that the single calibrated dial setting be an individually determined value, using the reported range for the appropriate manufacturer’s dose calibrator as a guide. (The limited range of reported Mark V values does not allow users to check their calibrated dial setting for reasonableness in this manner.) Specifically, we recommend, first, that each radiopharmacy establish a 90Y-calibrated dial setting based on NIST-supplied or NIST-traceable activity sources so that each activity source supplied to a medical facility can be used as a secondary reference standard and, second, that each medical facility determine its own calibrated dial setting based on the initial 90Y activity received from a commercial radiopharmacy or, alternatively, based on measurement of a NIST-traceable activity source in the same syringe geometry.
Using this approach, volume correction factors should not be necessary when measuring the activity of 90Y-ibritumomab tiuxetan at any volume in the range of 3–9 mL using the same type of syringe used in this study.
The type of syringe holder (e.g., T-handle or hook style dipper) used and inconsistent use of the protective well liner may cause further measurement variations. It is recommended that facilities always have the well liner installed during dose calibrator measurement of 90Y and not interchange syringe holders once they have established their calibrated 90Y dial setting.
CONCLUSION
This study demonstrated that, for accurate measurements, no adjustment is necessary for a dose calibrator dial setting when measuring different volumes of 90Y-ibritumomab tiuxetan activity prescriptions. Medical facilities need only establish their own calibrated dial setting for 90Y using their first prescription measurement based on the stated activity of the radiopharmacy.
Acknowledgments
This study was supported by IDEC Pharmaceuticals Corp.
Footnotes
Received Jun. 2, 2003; revision accepted Oct. 9, 2003.
For correspondence or reprints contact: Jeffry A. Siegel, PhD, Nuclear Physics Enterprises, 2202 Balsan Way, Wellington, FL 33414.
E-mail: siegelja{at}aol.com