Abstract
Cutaneous melanoma is often characterized by the presence of tumor-infiltrating lymphocytes (TILs). The degree of such infiltration and cell activation are considered significant prognostic factors reflecting the host’s immune response to the tumor; thus, patients with peritumoral infiltration may have a better prognosis and may also achieve a better response to interleukin-2 (IL2) immunotherapy. There is evidence that the expression of cluster designation (CD) 25 antigen (IL2 receptor [IL2R]) is a good marker of activity of T lymphocytes against melanoma cells. The aim of this study was to evaluate in vivo the binding of 99mTc-IL2 to lymphocytes infiltrating cutaneous melanoma and to determine whether such uptake correlates with immunologic and histologic data, thus providing useful prognostic information for IL2 therapy in patients with advanced disease. Methods: Thirty patients with cutaneous lesions suspected of being melanoma were studied. Planar γ-camera images over known tumor sites were acquired 1 h after the injection of 111–185 MBq of 99mTc-IL2. Tumor uptake of 99mTc-IL2 was measured as a target-to-background (T/B) radioactivity ratio. All patients underwent surgery, and histologic evaluation of the resected lesion was performed. The percentage of different peripheral blood lymphocyte subsets (CD3, CD4, CD8, CD16, CD25) and the percentage of IL2R-positive tumor cells on histologic sections were also measured. Results: At final histology, 21 lesions were found to be melanoma and 9 were classified as benign. In 15 of 21 (71%) melanomas and 2 of 9 (22%) benign cutaneous lesions, we found uptake of 99mTc-IL2. The calculated T/B ratios correlated significantly with the number of IL2R-positive TILs. Conclusion: 99mTc-IL2 scintigraphy provides a means of in vivo measurement of the extent of tumor infiltration of IL2R-positive cells, thereby providing valuable prognostic information for selection of patients who may benefit from IL2 immunotherapy.
Melanoma is a common tumor of the skin with a frequent rate of metastasis and generally poor prognosis, although if the tumor is diagnosed at an early stage, when no metastases are present, surgical treatment is often effective. It has been observed that many primary and secondary melanomas are characterized by the presence of tumor-infiltrating lymphocytes (TILs) and that a high level of infiltration correlates with a better prognosis (1,2).
The role of the immune response against melanoma is also confirmed by promising results obtained from the use of TILs as adjuvant therapy for stage III (regional lymph nodes) melanoma (3). On the basis of this observation, different immunomodulatory therapies have been developed in recent years, particularly those based on interleukin-2 (IL2) and interferon-α.
IL2 has been shown to be a potent in vitro stimulator of the generation of cytotoxic T lymphocytes and of lymphokine-activated killer cells not restricted by T-cell receptors, both of which kill in vitro tumor targets. Treatment with intravenous high-dose bolus IL2 has been shown to mediate objective responses in 15% of patients with metastatic melanoma (4). Approximately 8% of patients exhibit a complete response, which is durable in most cases (5). IL2 treatment is characterized, however, by high costs and the frequent occurrence of side effects. Among these, the capillary-leak syndrome with its associated hypotension is the most severe, requiring inotropic support for patients during treatment. There is a great need for accurate prognostic indicators that can identify a priori those patients who are most likely to benefit from biologic therapies. Several studies have attempted to identify predictive factors for IL2 response. For example, in a study of 81 patients receiving various IL2 regimens, increased C-reactive protein levels and the presence of visceral metastases were found to be negatively related to response (6). One of the best predictors of response is the limitation of metastases to only subcutaneous or cutaneous sites (7), and there is evidence that the expression of cluster designation (CD) 25 antigen is a good marker of antitumor activity of T lymphocytes on melanoma cells (8).
Scintigraphy using radiolabeled IL2 can detect in vivo the presence of activated organ-infiltrating lymphocytes and in particular those in an activated state expressing the IL2 receptors (IL2R). Scintigraphy with either 123I- or 99mTc-labeled IL2 has been used to image chronic inflammation-mediated disorders (9,10). The aim of this study was to determine whether 99mTc-IL2 can be used to detect peritumoral infiltration by activated lymphocytes in patients with melanoma undergoing surgical treatment. If this approach were to prove successful, the use of 99mTc-IL2 might, in future, be envisaged for the selection of patients with metastatic disease with significant levels of peritumoral lymphocytic infiltration who would be likely to respond to immunotherapy, for evaluation of the efficacy of such treatments, and for obtaining prognostic information on the likely disease outcome.
MATERIALS AND METHODS
Patients
Thirty consecutive patients with atypical pigmentary cutaneous lesions, undergoing surgical resection of the lesion, were enrolled in the study. All lesions were analyzed with respect to tumor invasiveness (Breslow depth, in millimeters, and Clark level, I–V) and lymphocytic infiltration (extent of the infiltrate, 0–3). Peripheral lymphocyte subsets (CD3, CD4, CD8, CD16, and CD25) were also measured in all patients.
The study was approved by the local Ethics Committee, and all patients gave their written informed consent before entering the study.
Evaluation of IL2R Expression on Melanoma Lesions
Analysis of IL2R (CD25) expression on histologic sections was performed for 17 of 21 melanoma lesions. Five-millimeter sections were freed of paraffin, and endogenous peroxidase activity was blocked with H2O2. Primary antibody antihuman IL2R (1:200 dilution; neoMarkers) was incubated for 1 h at room temperature followed by a secondary antibody incubation (biotinylated goat antimouse, 1:40 dilution) for 30 min at room temperature. Then, avidin biotin amplification (ABC kit; Dakocytomation) was added for 30 min. Incubation with AEC (3-amino-9-ethylcarbazole) chromogen kit (Sigma) at room temperature for 5–10 min produced a red reaction pigment. CD25-positive cells were counted at a magnification of 400× using a test grid with a 0.22-mm2 area. An average of 20 fields per section or several microscopic fields were counted until the SEM was <5% (11).
99mTc-IL2 Scintigraphy
All patients underwent 99mTc-IL2 scintigraphy. IL2 was radiolabeled using a 2-step technique as previously described (12). Briefly, the N3S bifunctional chelating agent S-tetrahydrofurfurylacetyl(thio-2,3,5,6 tetrafluorophenyl)adipylglycylglycine was first complexed with 99mTc at 80°C for 20 min at pH 1.8 and then conjugated to the protein at pH 9.5 and at room temperature for 45 min. After labeling, 99mTc-IL2 was purified from free 99mTc by reverse-phase chromatography using 0.1-g tC2 columns (Sep-Pak; Waters) eluted with acidified ethanol. The radiochemical purity of the protein was tested by instant thin-layer paper chromatography and trifluoroacetic acid precipitation (12). One hour after intravenous injection of 111 MBq (3 mCi) of 99mTc-IL2, static images over known lesions were acquired. Cutaneous lesions were classified as positive on the 99mTc-IL2 scan if planar images showed a detectable focus of radioactivity on visual analysis. Accumulation of 99mTc-IL2 into the lesions was also semiquantitatively calculated in the form of target-to-background (T/B) radioactivity ratios. For this purpose, an irregular (target) region of interest (ROI) was first drawn over the lesion; a second irregular ROI was then drawn around the first ROI, and the area between the 2 ROIs was considered background.
Statistical Analysis
Correlations between T/B ratios and immunologic and tumoral parameters were calculated using the Pearson coefficient for continuous variables and the Spearman ρ-coefficient for categoric variables. The Mann–Whitney test was used to evaluate the presence of differences between means of groups.
RESULTS
Histologic Features and Expression of CD25 in Melanomas and Benign Cutaneous Lesions
Of 30 patients with atypical pigmentary lesions, 21 were characterized as malignant melanoma and 9 as other benign cutaneous lesions. Melanomas were classified as macular (n = 11) or nodular lesions (n = 10) according to their size or their growth through the skin. Twenty-one of 21 melanoma lesions revealed a lymphocytic infiltration at histology. An inverse correlation was observed between the degree of lymphocytic infiltration and the Clark level (r = −0.552; P = 0.014). CD25 expression was detected on both TILs and on the melanoma cells themselves, but the intensity of staining was lower in the latter and was mainly intracytoplasmic, compared with membrane staining in lymphocytes. A variable level of CD25 expression was present on melanoma cells in 7 of 17 melanoma lesions studied (Table 1). CD25 expression on TIL was observed in 15 of 17 lesions analyzed, and the degree of IL2 receptor expression was again variable, ranging from 5% to 60% of infiltrating cells (Table 1). The degree of CD25 expression was significantly higher in nodular than in macular melanomas (28.89% ± 18.53% vs. 8.75% ± 11.26%, P = 0.007, Mann–Whitney). No correlation was observed between the degree of CD25 expression on melanoma cells and the degree of lymphocytic infiltration.
Immunologic, Histologic, and Scintigraphic Data on Patients with Melanoma
99mTc-IL2 Scintigraphy
No adverse reactions or side effects were observed after the intravenous administration of 99mTc-IL2. On visual analysis, 15 of 21 melanoma patients (71.4%) and 2 of 9 patients with benign lesions (22.2%) were judged as showing positive 99mTc-IL2 scan findings (Figs. 1–6). The degree of 99mTc-IL2 uptake by melanoma lesions, as assessed by T/B ratios, ranged from 1.00 to 1.81, and all visually positive lesions showed a T/B ratio higher than 1.1. In all patients with positive 99mTc-IL2 scintigraphy results, a significant degree of lymphocytic infiltration was detected at histology. Although CD25 expression was not measured in benign lesions, the 2 benign lesions (atypical nevi) with positive 99mTc-IL2 scintigraphy results also showed a significant lymphocytic infiltration at histology.
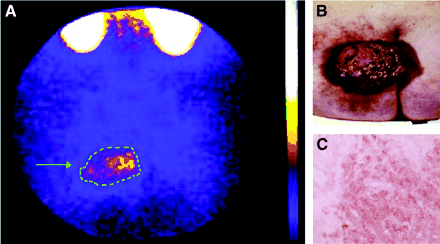
99mTc-IL2 scintigraphy (A) of a patient with a large melanoma (arrow) of the left gluteus (B). Despite the large size of the tumor, the distribution of radioactivity is inhomogeneous, reflecting patchy infiltration by activated T lymphocytes. T/B ratio was 1.81. (C) CD25 staining showed a mild positivity for neoplastic cells and a strong diffuse positivity for lymphocytes.
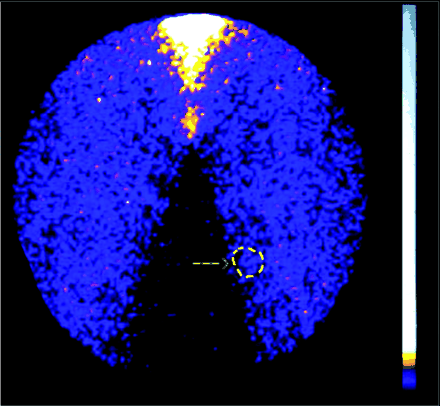
99mTc-IL2 scintigraphy (A) of a patient with a small melanoma near the right clavicle (B). Despite the small size of the tumor (green arrow), regional lymph nodes are also detectable (red arrow), reflecting active immune response inside primary and metastatic tumors. T/B ratio was 1.44. (C) CD25 staining showed a mild positivity for neoplastic cells and a strong diffuse positivity for lymphocytes.
99mTc-IL2 scintigraphy of a patient with a melanoma of the right foot. Moderate 99mTc-IL2 uptake (T/B = 1.14) is detectable in the tumor area (arrow).
99mTc-IL2 scintigraphy of a patient with a melanoma of the left foot. Strong 99mTc-IL2 uptake (T/B = 1.48) is detectable in the tumor area (arrow).
99mTc-IL2 scintigraphy of a patient with a melanoma of the left arm. No significant 99mTc-IL2 uptake (T/B = 1.00) is detectable in the tumor area (arrow).
99mTc-IL2 scintigraphy of a patient with an angioma of the left thigh. No significant 99mTc-IL2 uptake (T/B = 1.00) is detectable in the tumor area (arrow).
Correlations Between Scintigraphic Findings and Histologic Features
99mTc-IL2 accumulated in 11 of 15 lesions showing CD25 expression on TILs at histology. In addition, in 3 of 4 lesions judged as negative at scintigraphy, CD25 was expressed on fewer than 15% of TILs present. T/B ratios calculated on scintigraphic images significantly correlated with the percentage of CD25-positive lymphocytes on tissue sections (r = 0.745; P = 0.001; Fig. 7A). A weaker, although significant, correlation was also found if the percentage of CD25-positive lymphocytes was corrected for the intensity of lymphocytic infiltration (r = 0.512; P = 0.035). By contrast, no correlation was found between T/B ratios and CD25 expression on melanoma cells (Fig. 7B).
Correlation between T/B ratios at 99mTc-IL2 scintigraphy and the percentage of CD25-positive TILs (A) and melanoma cells (B).
Correlations Between Scintigraphic Findings and Peripheral Lymphocyte Subsets
Scintigraphic T/B ratios also correlated positively with the percentage of peripheral blood natural killer lymphocytes (CD16-positive: r = 0.654; P = 0.01), correlated inversely with the percentage of circulating CD3-positive lymphocytes (CD3-positive: r = −0.479; P = 0.033), and correlated inversely with the percentage of circulating CD4-positive T lymphocytes (r = −0.568; P = 0.09).
DISCUSSION
Our results show that 99mTc-IL2 accumulates in about 70% of melanomas with TILs expressing IL2 receptors (IL2R) and that the degree of 99mTc-IL2 uptake correlates with the percentage of IL2R-positive lymphocytes found at histology peritumorally (TILs). IL2R were also detected on the surface of melanoma cells, but this finding did not correlate with the T/B ratio calculated after 99mTc-IL2 scintigraphy. A possible explanation for these results could be the lower density of CD25 expression on melanoma cells (as detected on histologic studies) or the presence of mainly intracytoplasmic antigen. However, another possible explanation is the status of the IL2R on the tumor cells. In vivo, the uptake of radiolabeled IL2 represents mainly its binding to high-affinity IL2 receptors, which are trimers composed of the α-, β-, and γ-chains. The antibody used for immunohistochemical studies detects only the α-chain of this receptor (CD25), to which IL2 binds with low affinity (13). Although the α-chain of the IL2R is expressed in a high percentage of several tumor cells, the β-chain has been detected only in a smaller number of cases (14) and high-affinity IL2R is rare on melanoma cells. On the other hand, TILs are equipped with a fully functional IL2R system, thus explaining TIL activation and expansion during immunotherapy with IL2 (15). High-affinity IL2R expression on TILs represents a marker of lymphocyte activation, which occurs only when lymphocytes recognize certain specific melanoma antigens that have been classified as tumor-associated testis-specific antigens, melanocyte differentiation antigens, or mutated or aberrantly expressed antigens (16). Thus, the level of IL2R expression on TILs could represent a more objective marker of lymphocyte activity against tumors than does the absolute number of TILs present in the lesion.
99mTc-IL2 scintigraphy could therefore be used in patients with stage III or IV melanomas who are potential candidates for immunotherapy. The presence or absence of 99mTc-IL2 accumulation in lymph nodes or distant metastases could provide useful prognostic information on the possible effect of such treatment. Significant correlations were also found between the degree of 99mTc-IL2 uptake and the number of CD16, mainly natural killer, cells (positive correlation) and with the percentage of CD3-positive and CD4-positive circulating lymphocytes (inverse correlation). Although interpretation of such correlations is difficult, CD16 expressed on human natural killer cells represents signal-transducing molecules that, on ligand binding, induce expression of surface activation molecules (such as IL2R) and production of cytokines relevant to natural killer cell biology and function (17). Thus, the percentage of IL2R-positive TILs could itself be influenced by the number of circulating CD16-positive cells.
CONCLUSION
These results suggest a possible role for 99mTc-IL2 scintigraphy in the management of patients affected by cutaneous melanoma. In particular, this technique could provide unique information on the biologic properties of the tumor that would influence its response to therapeutic interventions. To test this hypothesis, we are planning a longitudinal study to correlate the results of IL2 immunotherapy and 99mTc-IL2 scintigraphy.
Acknowledgments
The authors thank Dr. Giampaolo Nardi and Dr. Paolo Giacalone for their helpful collaboration in the radiolabeling of IL2. This work was partially supported by research grants from the Italian Ministry of Education, Universities, and Research. The authors also thank Chiron Italy and the Associazione Romana Ricerca Dermatologica for kind collaboration in the study.
Footnotes
Received Jan. 13, 2004; revision accepted May 21, 2004.
For correspondence or reprints contact: Alberto Signore, MD, Nu.M.E.D. Group, Dipartimento Scienze Cliniche, Policlinico Umberto I, 00161 Roma, Italy.
E-mail: alberto.signore{at}uniroma1.it