Abstract
The Nuclear Regulatory Commission (NRC) regulations that govern release of patients administered radioactive material have been revised to include dose-based criteria in addition to the conventional activity-based criteria. A licensee may now release a patient if the total effective dose equivalent to another individual from exposure to the released patient is not likely to exceed 5 mSv (500 mrem). The result of this dose-based release limit is that now many patients given therapeutic amounts of radioactive material no longer require hospitalization. This article presents measured dose data for 26 family members exposed to 22 patients treated for non-Hodgkin’s lymphoma with 131I-anti-B1 antibody after their release according to the new NRC dose-based regulations. Methods: The patients received administered activities ranging from 0.94 to 4.77 GBq (25–129 mCi). Family members were provided with radiation monitoring devices (film badges, thermoluminescent or optically stimulated luminescent dosimeters, or electronic digital dosimeters). Radiation safety personnel instructed the family members on the proper wearing and use of the devices. Instruction was also provided on actions recommended to maintain doses to potentially exposed individuals as low as is reasonably achievable. Results: Family members wore the dosimeters for 2–17 d, with the range of measured dose values extending from 0.17 to 4.09 mSv (17–409 mrem). The average dose for infinite time based on dosimeter readings was 32% of the predicted doses projected to be received by the family members using the NRC method provided in regulatory guide 8.39. Conclusion: Therapy with 131I-anti-B1 antibody can be conducted on an outpatient basis using the established recommended protocol. The patients can be released immediately with confidence that doses to other individuals will be below the 5-mSv (500 mrem) limit.
In 1997, the Nuclear Regulatory Commission (NRC) amended its regulations concerning criteria for the release of patients who have been administered radioactive material (1). The new criteria authorize patient release according to a dose-based limit (5 mSv to the maximally exposed individual) rather than the traditional activity-based limit (<1.11 GBq [30 mCi] or <0.05 mSv [5 mrem/h] at 1 m). The dose-based limit better expresses the primary concern of the NRC for public health and safety. This concern is reflected in a revised version of 10 Code of Federal Regulations (CFR) 35.75, which governs the release of patients containing radioactive materials; guidance is given in regulatory guide 8.39 (2). Compliance with this dose limit may be shown by licensees in 3 ways: use of a default table of administered activity, use of a default table of patient dose rates, or use of patient-specific dose calculations. A regulatory analysis (3) of the new dose-based limit concluded that the new standard is acceptable according to current radiation protection principles, resulting in fewer hospitalizations, and therefore significantly reduces national health care costs; in addition, earlier release benefits patients and their families personally and psychologically.
Before the NRC rule change, most radionuclide treatment protocols required extended patient hospitalization. This requirement, though intended to protect family members and others who would otherwise be in close contact with the patient, added to the effort, cost, and inconvenience of this treatment. In many cases, therapies were performed as inpatient solely to comply with regulations and not for medical reasons. In some instances, the previous limit coerced physicians to administer less radioactivity than they would have liked so that hospital stays could be avoided (4). Under the new regulations, many patients can now be immediately released from the hospital or clinic after therapy with radionuclides (5–8). Patient-specific calculations have indicated that all patients receiving 131I-anti-B1 monoclonal antibody (Bexxar, tositumomab and 131I-tositumomab; Corixa Corp., South San Francisco, CA), an investigational new therapy for B-cell non-Hodgkin’s lymphoma (9–11), are now releasable. Therefore, the new regulations permit 131I-anti-B1 antibody therapy to be conducted on an outpatient basis using the established recommended protocol (5).
Although the patient who has received 131I-anti-B1 antibody is releasable, it is important to determine whether other individuals exposed to the released patient are receiving doses < 5 mSv (500 mrem). Direct measurements are the best way to determine the dose any individual is likely to receive on the basis of the realities of daily living. In most cases, the maximally exposed individual will be a close family member. Generally, one must assume that such individuals will have little or no knowledge of radiation safety and thus require some instructions to limit their potential exposure. Although the NRC has provided patient release criteria (2), guidance on instructing these patients to keep the radiation dose to others as low as is reasonably achievable (ALARA) is limited. Recently, more guidance has been provided in the literature (5,6,12,13). Therefore, this study was conducted to determine the radiation doses received by maximally exposed members of the general public (e.g., family members) from patients who received therapeutic doses of 131I-anti-B1 antibody as an outpatient treatment and to determine whether the instructions provided to maintain doses ALARA were adequate. The family members were provided with radiation monitoring devices (film badges, thermoluminescent or optically stimulated luminescent dosimeters (OSLs), or electronic digital dosimeters) to measure their radiation doses and also to confirm that these doses were below regulatory limits. Instructions were provided on actions recommended to keep doses to potentially exposed individuals ALARA. The dose measurement results of the radiation monitoring devices worn by the family members confirm the appropriateness of and patient compliance with the instructions provided.
MATERIALS AND METHODS
Patients
Twenty-two patients received intravenous radioimmunotherapy with 131I-anti-B1 monoclonal antibody. Patients were administered a therapeutic amount calculated to deliver a nonmyeloablative total-body absorbed dose (30–75 cGy) as part of several different clinical research protocols. The administered therapy dose was based on the patient’s total-body residence time, which was determined from an initial dosimetric study (14).
Patient-Specific Dose Calculation
According to the regulatory guidance, patients may be released on the basis of specific conditions. The following equations were used to calculate the total effective dose equivalent to individuals exposed to the patient for an infinite time (derivations of these equations are discussed in the Appendix):
On the basis of administered activity:
 Eq. 1
On the basis of the patient’s dose rate:
Eq. 1
On the basis of the patient’s dose rate:
 Eq. 2
where D(∞) is the total effective dose equivalent (millisieverts) to the maximally exposed individual over an infinite time, Q0 is the administered activity (megabecquerels), Teff is the patient’s total-body effective half-time (days) determined by measurements after a tracer dose, Teff is 0.693 × τ (residence time) under the condition of modeling whole-body retention as a single exponential, and Dr is the dose rate (mSv/h) at 1 m from the patient immediately after therapeutic administration.
Eq. 2
where D(∞) is the total effective dose equivalent (millisieverts) to the maximally exposed individual over an infinite time, Q0 is the administered activity (megabecquerels), Teff is the patient’s total-body effective half-time (days) determined by measurements after a tracer dose, Teff is 0.693 × τ (residence time) under the condition of modeling whole-body retention as a single exponential, and Dr is the dose rate (mSv/h) at 1 m from the patient immediately after therapeutic administration.
The release criteria calculated using the administered activity (Eq. 1) are more conservative than those calculated using the patient’s dose rate (Eq. 2), because no attenuation of the radiation by the body is considered. With the release limit of D(∞) < 5 mSv (500 mrem), Equations 1 and 2 can be rearranged as follows to determine maximum administered activity or patient dose rate for patient release (i.e., either Eq. 3 or Eq. 4 must be true to allow release):
 Eq. 3
Eq. 3
 Eq. 4
Eq. 4
These calculations take into account internal dose contribution and are based on conservative assumptions given in regulatory guide 8.39 (2). For example, regulatory guide 8.39 assumes that for the first 8 h after administration of radioiodine, 80% of the radioactivity is not voided from the urinary bladder (e.g., eliminated solely by the 8-d physical decay of 131I) and that the occupancy factor (the fraction of time that the maximally exposed individual is within 1 m of the patient) is 0.75 for this initial period.
If the actual administered activity is less than the activity determined according to Equation 3, then the patient is releasable according to the new NRC regulations. Equation 3 involves the use of only a single patient-specific factor (i.e., effective half-time), which must be included in the patient’s record at the time of release. Equation 4 was also used to determine the releasibility of the patient. In this case, a second patient-specific factor, the patient’s dose rate at 1 m, which accounts for attenuation, must also be included in the patient’s record at the time of release. The dose rate is measured after the therapeutic administration. All the calculations assume the use of an occupancy factor of 0.25 after the initial 8-h nonvoiding period. The occupancy factor is the fraction of time that an individual is assumed to be 1 m away from the released patient. If there is justification for using a lower occupancy factor of 0.125, or if a higher occupancy factor of 0.5 or more is indicated, then the calculated values must be changed accordingly (5,7).
For the 131I-anti-B1 antibody protocol, data indicate that a more appropriate assumption is that an initial nonvoiding period of 3 h can be used, instead of the 8-h period suggested by the NRC. A 3-h period is more appropriate because it has been shown to be a conservative estimate for the time of the first voiding of the urinary bladder (15) and because it is consistent with the analysis performed on 109 131I-anti-B1 antibody patient studies (6). The conservative nature of this 3-h assumption is further supported by the fact that 131I-anti-B1 antibody is absorbed instantaneously because of its intravenous administration, whereas regulatory guide 8.39 assumed oral administration. Additionally, for this initial nonvoiding period, it makes sense to account for 100% of the administered activity and not the 80% recommended in regulatory guide 8.39.Using these assumptions and the fact that R/h = Γ Q0/r2, the dose over an infinite time to the exposed individual becomes:
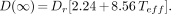 Eq. 5
Eq. 5
This equation was also used to project the dose for infinite time in this study.
Guidelines
If the calculations indicate that the patient is releasable, one then determines whether the patient can actually be released. Patients containing >1.22 GBq (33 mCi) 131I (or with a dose rate > 0.07 mSv/h [7 mrem/h] at 1 m) can be released if one can show that no individual who comes into contact with the patient is likely to receive a dose > 5 mSv. The release is dependent on the circumstances of each patient. Interviewing the patient and using that information to determine whether the patient may be released are essential. Factors to consider include the patient’s ability to understand and willingness to follow written instructions, the patient’s ability to care for himself or herself, the patient’s ability to refrain from returning to work if necessary, the patient’s exposure to others while returning home after treatment, and the presence of urinary incontinence. The form that we used to interview patients is shown in Figure 1. Once the patient interview is completed, the responsible physician or radiation safety officer evaluates whether the patient can be released. If the determination is affirmative, discharge instructions are given to the patient.
Form used to determine whether patient can be released from hospital after radioimmunotherapy with 131I-anti-B1 antibody. S/N = serial number.
Instructions to Patients and Caregivers
Once the release has been determined, the patient must be provided with written instructions to comply with the provisions of 10 CFR 35.75(b). The instructions and all related discussions must be in a simple and clear format so that the patient can understand their importance. Specific instructions were developed to address the unique requirements of patients treated with the 131I-anti-B1 antibody to maintain exposures ALARA to other individuals.
Patient discharge instructions for various activities (e.g., using public transportation, attending to personal hygiene, and maintaining distance from others) were developed using exposure data obtained from patients who had been treated with 131I-anti-B1 antibody and confined under the old release regulations and by making assumptions about the distances at which individuals typically interact with each other in various social situations. A diary was kept by the maximally exposed individual to record the times that the radiation monitoring device was worn and the interactions with the patient.
The radiation safety discharge instructions were provided to and discussed with the patients and caregivers (if possible) by the nuclear medicine physician or radiation safety personnel before the release of the patient. Any questions about radiation safety issues were answered at that time. One copy of these written instructions was provided to the patient, and a second copy was maintained in the patient’s files.
Radiation Monitoring
Family members received film badges, thermoluminescent dosimeters (TLDs), OSLs, or electronic digital dosimeters. In most cases, the caregiver was given more than a single type of device. Radiation safety personnel taught the caregivers how to wear and use the devices. The caregivers were also asked to log their activities and resultant exposures to verify the appropriateness of the discharge instructions and to confirm that the radiation doses to the family members were below the regulatory limits. The readings were also compared with the theoretic doses over an infinite time predicted by the patient-specific calculations.
Data Analysis
All radiation monitoring devices were processed on return. Diaries of the direct-reading dosimeters were reviewed, and the readings were transferred to spreadsheets for subsequent analyses. The final dosimeter reading was used to calculate the predicted dose over an infinite time based to the maximally exposed individual using the following equation:
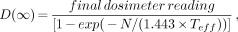 Eq. 6
where D(∞) is the total effective dose equivalent (millisieverts) to the maximally exposed individual and N is the number of days the individual was monitored. This “measured” dose for infinite time was compared with the doses for infinite time predicted by Equations 1, 2, and 5.
Eq. 6
where D(∞) is the total effective dose equivalent (millisieverts) to the maximally exposed individual and N is the number of days the individual was monitored. This “measured” dose for infinite time was compared with the doses for infinite time predicted by Equations 1, 2, and 5.
RESULTS
Twenty-two non-Hodgkin’s lymphoma patients were entered into several dose-escalating radioimmunotherapy clinical trials, some of which included chemotherapy and bone marrow transplantation. These patients received therapeutic doses of 131I-anti-B1 antibody ranging from 0.94 to 4.77 GBq (25–129 mCi), resulting in total-body absorbed doses of 30–75 cGy (30–75 rad). The effective half-life of total-body clearance as determined from the dosimetry study ranged from 46 to 85 h. The dose rates at 1 m before patient discharge after the therapeutic administration ranged from 0.03 to 0.18 mSv/h (3–18 mrem/h) (Table 1). All but 1 patient were found to be immediately releasable on the basis of administered activity or dose rate. On the basis of the measured dose rate and application of Equation 4, patient 22 was told to remain in the clinic for 1 h before release. This patient would have been immediately releasable using Equation 5 (using a 3-h nonvoiding period). The radiation doses to family members ranged from 0.17 to 4.09 mSv (17–409 mrem) for indirect-reading dosimeters (e.g., TLDs and OSLs), with monitoring periods ranging from 3 to 17 d (mean, 8.2 d). Direct-reading dosimeter exposures ranged from 0.10 to 3.54 mSv (10–354 mrem), with monitoring periods ranging from 2.1 to 17 d (mean, 6.5 d).
Patient Data
The predicted doses for infinite time from these patients were calculated using Equations 1, 2, and 5 (Table 2). The predicted dose for infinite time using the maximum dosimeter reading for a family member was also calculated using Equation 6. All the doses over an infinite time based on dosimeter readings using Equation 6 (measured doses for infinite time) were below the 5-mSv (500 mrem) regulatory limit. Table 3 summarizes the predicted versus measured doses for infinite time. The average measured dose for infinite time was found to be 1.68 mSv (168 mrem), with an SD of 1.08 mSv. The median was 1.51 mSv (151 mrem). The measured doses for infinite time as a percentage of the predicted doses for infinite time based on Equations 1, 2, and 5 for all patients were found to average 32%, 47%, and 58%, respectively (Table 4). Results are also summarized in Tables 3 and 4 for the 7 patients receiving a 75-cGy total-body dose, because this is the expected treatment dose for this protocol. For the patients receiving 75 cGy, the average measured dose for infinite time was found to be 2.02 mSv (202 mrem), with a median of 2.27 mSv (227 mrem). The measured doses for infinite time as a percentage of the predicted doses for infinite time based on Equations 1, 2, and 5 were found to be significantly lower, averaging 35%, 53%, and 67%, respectively (Table 4).
Predicted vs. Measured Dose over an Infinite Time
Summary of Predicted and Measured Doses for Infinite Time
Comparison of Measured Dose for Infinite Time vs. Predicted Dose for Infinite Time
DISCUSSION
The early release of these patients should lower health care costs and provide emotional benefits to the patients and their families and may improve outcome and lead to more effective health care. Health care professionals caring for patients in hospitals (e.g., the nursing staff) will receive a much lower radiation dose because of their decreased exposure to this type of patient. A potential disadvantage to releasing patients is that certain individuals exposed to them could receive a higher dose than if the patient remained hospitalized longer; however, if the patient is given appropriate instructions, that dose should be modest and below the limit set by the NRC. The 20 NRC states are governed by the new regulations. However, the 30 agreement states are not required to follow these recommendations and would therefore have to amend their regulations to release patients on the basis of these new criteria. To date, at least 20 of the 30 agreement states have already amended their regulations or granted individual institutions variances that permit outpatient release.
The fact that the new regulations are dose-based rather than activity-based is an advantage because this change standardizes the dose for release among different radionuclides, each of which is characterized by a different half-life and spectrum of emissions. Patients can now be released regardless of how much administered activity they received, as long as the total dose to any individual is not likely to exceed 5 mSv (500 mrem), which is approximately 1.5 times the exposure the average American receives annually from natural background radiation.
The preference for this dose-based approach for patient release was expressed more than 30 y ago, as indicated by the following statement in NCRP report 37, from 1970 (16): “Since the exposure rates and half-lives of various radionuclides differ greatly, a more meaningful basis for release from the hospital is the possible exposure to other individuals with whom the patients are likely to associate.”
When the predicted dose for infinite time to the maximally exposed individual is calculated, Equation 1 (administered activity) will always yield a greater dose than Equation 2 (patient dose rate) because Equation 1 conservatively assumes a point source geometry with no consideration for body attenuation. Likewise, Equation 2 will yield a more conservative dose for infinite time than Equation 5 because of the differences in the initial nonvoiding period. Although less conservative, Equation 5 should be used in predicting dose for infinite time for 2 reasons: first, because the initial 3-h nonvoiding period is a more appropriate model for this protocol and, second, because our results show that the measured dose for infinite time will be considerably less than the predicted dose for infinite time the maximally exposed individual will receive (e.g., measured dose was 33% less than predicted dose for the patients receiving 75 cGy).
For patients 14 and 15, who had the highest measured dose for infinite time to the maximally exposed individual, the monitoring was shared by more than a single person. On the basis of these patients’ travel and housing situations (i.e., exposure to various individuals), we determined that having more than a single person use the dosimeter would better approximate the dose to the maximally exposed individual. In both cases, the measured dose for infinite time was less than the 5-mSv limit.
For patient 11, the monitoring period was only 3 d. The monitored individual received 0.79 mSv during this period; however, projected out to infinite time, the resulting dose is 1.71 mSv. This dose is higher than what Equation 2 (1.22 mSv) or Equation 5 (1.02 mSv) predicts. This patient was to receive conventional chemotherapy shortly after the therapeutic administration, and in this situation the patient and caregiver spent more time together than usual during this short time.
CONCLUSION
Twenty-two patients were treated for non-Hodgkin’s lymphoma using 131I-anti-B1 antibody. After release of the patients, 26 family members were monitored for radiation exposure. All radiation doses received by these nonoccupational caregivers were below the regulatory limit of 5 mSv (500 mrem). These results indicate that the written instructions and the radiation safety counseling were effective in keeping exposures ALARA. Therefore, treatment with 131I-anti-B1 antibody for non-Hodgkin’s lymphoma can be performed on an outpatient basis.
APPENDIX
NRC Default Tables and Patient-Specific Calculations
Regulatory guide 8.39 (2) provides default tables with values authorizing patient release based on administered activities or 1-m patient dose rates for a variety of radionuclides. The values calculated for both tables are based solely on the physical half-life of the radionuclide (i.e., no biologic elimination is assumed). The equation used to calculate these release values is essentially the same as introduced in 1970 by National Council on Radiation Protection and Measurements report no. 37 (16) with the exception of the occupancy factor. The selection of an occupancy factor of 0.25 at 1 m for estimating the 131I dose to an individual from exposure to a released patient is based on the professional judgment of time–distance combinations that are likely after instructions to minimize time near the patient.
Use of the physical half-life, not the effective half-life, of the radionuclide assumes that the body retains the radionuclide (e.g., 131I) until it is fully decayed and that none is cleared through biologic processes. Clearly, this is not true: biologic processes do affect the clearance of radionuclides. Patients receiving 131I therapy do not retain radioactivity for the physical half-life of the radionuclide. Rather, patients eliminate 131I more quickly because of biologic elimination. As a result, the patient-specific dose calculations, which take into account both the physical and the biologic half-life (i.e., the effective half-life) of the radionuclide, are more complete and appropriate than the NRC default tables in calculating the dose an individual will likely receive if exposed to a patient treated with 131I (7). Because the default tables do not take into account the biologic elimination of the radionuclide, their use will overestimate the dose an individual would receive if exposed to a patient treated with 131I. Using a patient-specific dose calculation provides a more complete and appropriate estimation of dose.
Direct measurements are the best way to obtain the dose any individual is likely to receive under realistic exposure conditions. Three previous studies (17–19) measured doses to family members from patients who were released after treatment of thyroid cancer or hyperthyroidism with <1.11 GBq (30 mCi) 131I. These studies showed that use of only the physical half-life in calculations will overestimate radiation doses received by family members and suggested that the patient-specific dose calculation will be conservative. These data are summarized in Table 1A. On the basis of these 3 studies, a regulatory analysis (3) concluded that the revised NRC patient-release rule provides an adequate level of protection, with a significant margin of safety for patients who make a reasonable effort to follow instructions. Therefore, both professional judgment and empiric measurements support the validity of using the patient-specific dose calculation in determining the maximum likely radiation dose to another individual. The radiation dose predicted by the calculation is usually significantly higher than the dose obtained by direct measurements with film badges or TLDs worn by the family members of the patients.
Comparison of Measured Dose with Dose Predicted Using Physical Half-Life and Biologic Elimination
Regulatory guide 8.39 allows the licensee to release patients on the basis of patient-specific calculations, including using the biologic or effective half-life. The procedure for calculating doses based on patient-specific factors is given in Appendix B of the regulatory guide. To account for the time for 131I to be absorbed from the stomach and the holdup of iodine in the urine while in the bladder, the regulatory guide conservatively makes the following assumptions: during the first 8 h after administration, 80% of the 131I is removed from the body by only the physical decay of 131I, and the occupancy factor for this 8-h nonvoiding period is assumed to be 0.75 (0.25 after this period).
The University of Nebraska Medical Center has performed several 131I-anti-B1 antibody therapies since late 1996. In all cases, a monoexponential clearance rate has been observed. Using the assumptions of the regulatory guide for the initial 8-h period and a monoexponential clearance after this nonvoiding period, and taking into account the internal dose contribution from 131I (Eq. B-6 in the regulatory guide), the following equation based on a point source geometry determines the dose for infinite time to the maximally exposed individual:
 Eq. 1A
Eq. 1A

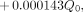 where D(∞) is the dose for an infinite time to the maximally exposed individual (millisieverts); Q0 is the administered activity (megabecquerels); 34.6 is a conversion factor of 24 h/d divided by ln2 (resulting from integration); Γ is the γ-ray constant, which is 0.595 mSv-cm2/MBq-h for 131I; E1 is the occupancy factor for the first 8 h, or 0.75; E2 is the occupancy factor after 8 h, or 0.25; Tp is 8.04 d; Teff is the effective half-life (days) based on the patient’s dosimetric dose (e.g., the initial 185-MBq [5 mCi] dose administered to calculate the activity required for that patient’s therapy); and 0.000143 is a factor derived from regulatory guide 8.39.When multiplied by Q0, this factor gives the internal dose contribution in millisieverts. On simplification, the equation for dose for infinite time becomes:
where D(∞) is the dose for an infinite time to the maximally exposed individual (millisieverts); Q0 is the administered activity (megabecquerels); 34.6 is a conversion factor of 24 h/d divided by ln2 (resulting from integration); Γ is the γ-ray constant, which is 0.595 mSv-cm2/MBq-h for 131I; E1 is the occupancy factor for the first 8 h, or 0.75; E2 is the occupancy factor after 8 h, or 0.25; Tp is 8.04 d; Teff is the effective half-life (days) based on the patient’s dosimetric dose (e.g., the initial 185-MBq [5 mCi] dose administered to calculate the activity required for that patient’s therapy); and 0.000143 is a factor derived from regulatory guide 8.39.When multiplied by Q0, this factor gives the internal dose contribution in millisieverts. On simplification, the equation for dose for infinite time becomes:
 Eq. 2A
Eq. 2A
Using the patient dose rate at 1 m (Dr), the equation for dose for infinite time becomes:
 Eq. 3A
where Dr is the patient dose rate at 1 m (mSv/h). The administered activity and dose rate at which the patient may be released can be determined by setting the dose for infinite time in Equations 2A and 3A to 5 mSv (500 mrem) and solving for the corresponding parameter as follows:
Eq. 3A
where Dr is the patient dose rate at 1 m (mSv/h). The administered activity and dose rate at which the patient may be released can be determined by setting the dose for infinite time in Equations 2A and 3A to 5 mSv (500 mrem) and solving for the corresponding parameter as follows:
 Eq. 4A
Eq. 4A
 Eq. 5A
Eq. 5A
Acknowledgments
The authors thank Subhash Pakinar, MD, Kay Devish, Pam Cox, Beverly Schmidt, and Susan Blummel for helping with the study. This study was supported by Corixa Corp.
Footnotes
Received Jun. 2, 2000; revision accepted Sep. 18, 2000.
For correspondence or reprints contact: Frank J. Rutar, MS, University of Nebraska Medical Center, 985480 Nebraska Medical Center, Omaha, NE 68198-5480.








