Abstract
A methodology was developed determining patient releasability after radioimmunotherapy with tositumomab and 131I-tositumomab for the treatment of non-Hodgkin’s lymphoma. Methods: Dosimetry data were obtained and analyzed after 157 administrations of 131I-tositumomab to 139 patients with relapsed or refractory non-Hodgkin’s lymphoma. Tositumomab and 131I-tositumomab therapy included dosimetric (low activity) and therapeutic (high activity) administrations. For each patient, the total-body residence time was calculated after the dosimetric administration from total-body counts obtained over 6 or 7 d and was then used to determine the appropriate therapeutic activity to deliver a specific total-body radiation dose. Patient dose rates at 1 m were measured immediately after the therapeutic infusion. Patient-specific calculations based on the measured total-body residence time and dose rate for 131I-tositumomab were derived to determine the patient’s maximum releasable dose rate at 1 m, estimated radiation dose to maximally exposed individuals, and the amount of time necessary to avoid close contact with others. Results: The mean administered activity (±SD), determined by dosimetry studies for each patient before therapy, was 3,108 ± 1,073 MBq (84 ± 29 mCi) (range, 1,221 ± 5,957 MBq [33–161 mCi]). Immediately after treatment, the mean measured dose rate (±SD) at 1 m was 0.109 ± 0.032 mSv/h (10.9 ± 3.2 mrem/h; range, 0.04–0.24 mSv/h [4–24 mrem/h]). The measured dose rates were 60% (range, 37%–90%; P < 0.0001) of the theoretic dose rates from a point source in air predicted using the dose equivalent rate per unit activity of 131I (5.95 × 10−5 mSv/MBq h [0.22 mrem/mCi h] at 1 m). The mean estimated radiation dose to the maximally exposed individual was 3.06 mSv (306 mrem) (range, 1.95–4.96 mSv [195–496 mrem]). On the basis of current regulatory patient-release criteria, all 131I-tositumomab–treated patients were determined to be releasable by comparing the dose rate at 1 m with a predetermined maximum releasable dose rate. Detailed instructions were provided to limit family members’ exposure. Conclusion: A methodology has been developed for the release of patients administered radioactive materials based on the new Nuclear Regulatory Commission regulations. This approach uses a patient-specific dose calculation based on the measured total-body residence time and dose rate. This analysis shows the feasibility of outpatient radioimmunotherapy with tositumomab and 131I-tositumomab.
Radioimmunotherapy is a promising treatment modality for patients with B-cell malignancies and other cancers. Radioimmunotherapy with tositumomab and iodine I 131 tositumomab (Corixa Corp., South San Francisco, CA) is under clinical investigation for the treatment of patients with low-grade and transformed low-grade non-Hodgkin’s lymphoma. This therapeutic regimen is a combination radioimmunotherapeutic composed of an unlabeled monoclonal antibody (tositumomab) and a radiolabeled monoclonal antibody (131I-tositumomab). Clinical trials have already shown that tositumomab and 131I-tositumomab therapy commonly produces durable responses in patients with low-grade and transformed low-grade non-Hodgkin’s lymphoma (1–4), suggesting that non-Hodgkin’s lymphoma patients will benefit from the expanded application of tositumomab and 131I-tositumomab therapy.
Tositumomab and 131I-tositumomab therapy for non-Hodgkin’s lymphoma is customized to the individual patient. The patient-specific therapeutic activity of 131I-tositumomab is determined through pretherapy dosimetry (low activity) studies. Because the thyroid is blocked by supersaturated potassium iodide before therapy, any 131I that dissociates from tositumomab rapidly enters the bloodstream and is excreted in the urine. Therefore, the clearance rate (i.e., residence time) of 131I is primarily dependent on the residence time of the radiolabeled antibody in the individual patient (5). After a 1-h preadministration of unlabeled tositumomab (475 mg on day 0) to saturate CD20 on circulating B-cells and maximize tumor targeting, patients receive a trace-labeled dosimetric (<185 MBq [<5 mCi]) administration of 131I-tositumomab. Total-body gamma-camera counts obtained 3 times over 6–7 d are used to determine the residence time of the radiolabeled antibody in the body. The therapeutic activity, based on the total-body residence time, is then calculated to ensure that the patient receives the maximum tolerated whole-body absorbed dose of 75 cGy—that is, the absorbed dose shown previously to produce acceptable hematologic (i.e., bone marrow) toxicity (2–4).
Until recently, radioimmunotherapy typically required a hospital stay of several days to comply with the applicable patient-release regulations. However, in 1997 the Nuclear Regulatory Commission (NRC) amended the regulations relating to the criteria for the release of patients administered radioactive material (6). The new criteria for patient release are based on limiting the total effective dose equivalent (TEDE) to <5 mSv (<500 mrem) for the maximally exposed individual. Previously, patient release was based on the retained activity (<1,110 MBq [<30 mCi]) or the dose rate at 1 m (<0.05 mSv/h [<5 mrem/h]). The new regulations presented in a revision of Title 10, Part 35.75, of the Code of Federal Regulations (10 CFR 35.75) (6) and in Regulatory Guide 8.39: Release of Patients Administered Radioactive Materials (7), state that licensees may demonstrate compliance with the TEDE limit of 5 mSv by (a) using a default table for 131I within Regulatory Guide 8.39 for releasable activity, 1,221 MBq (33 mCi), or releasable dose rate at 1 m, 0.07 mSv/h (7 mrem/h); or (b) performing a patient-specific dose calculation (calculating the TEDE to individuals exposed to the patient using the patient’s measured residence time for the radiopharmaceutical and dose rate). A regulatory analysis of the new dose-based limit concluded that it is safe, results in shorter hospital stays, reduces health care costs, and has personal and psychologic benefits for the patients and their family members (8).
Previous analyses of the patient-specific calculation approach for the determination of the TEDE to maximally exposed individuals for 131I-tositumomab–treated non-Hodgkin’s lymphoma patients treated on an inpatient basis revealed that all patients evaluated were releasable immediately after treatment under the new NRC regulations (9,10) and that the maximum exposure to radiation of any individual did not exceed 3.7 mSv (10). A study performed at the University of Nebraska Medical Center (11) using direct dose measurements showed that caregivers of patients undergoing tositumomab and 131I-tositumomab therapy received between 0.1 and 3.54 mSv, well below the applicable NRC limits, corroborating the usefulness of the patient-specific dose calculation. Similar studies of family members of patients undergoing outpatient 131I therapy for thyroid carcinoma and hyperthyroidism also indicated that if proper instructions to minimize exposure were followed, all primary caregivers were exposed to <5 mSv of radiation (12–14). Therefore, the development of practical, patient-specific calculations that estimate the maximum likely radiation exposure of the maximally exposed individual and provide a basis for the determination of patient release may expand the availability of outpatient radioimmunotherapy. Outpatient radioimmunotherapy would also improve the quality of life of patients by allowing them to return home immediately after therapy.
The objective of this study was to develop a practical methodology for the determination of the release of patients undergoing tositumomab and 131I-tositumomab therapy. Patient-specific calculations and measurements were developed to determine releasability and ensure that the radiation exposure of the maximally exposed individual will likely not exceed 5 mSv.
MATERIALS AND METHODS
Patients and Treatments
Data were analyzed after 157 therapeutic administrations of 131I tositumomab to 139 patients for the treatment of relapsed or refractory non-Hodgkin’s lymphoma at 2 sites (the University of Michigan Medical Center, Ann Arbor, MI, and the University of Nebraska Medical Center, Omaha, NE) (2–4). These patients were enrolled in phase I/II and phase II studies conducted at the University of Michigan Medical Center, a multicenter phase II study, and a multicenter phase III study. Patients underwent dosimetry studies, followed 7–14 d later by the therapeutic administration. One day before and throughout the dosimetry study and the therapeutic administration, the thyroid was blocked by daily administration of supersaturated potassium iodide. For the dosimetric administration, most patients received a 450-mg infusion of unlabeled tositumomab followed by a 35-mg infusion of trace-labeled 131I-tositumomab (185 MBq 131I). After the dosimetric administration, thyroid probes or total-body gamma-camera counts were obtained at least 3 times over the next 6–7 d to determine the patient-specific total-body residence time of the radiolabeled antibody. The residence time, in conjunction with a mass-adjusted S value based on the patient’s height and weight, was used to determine the activity of 131I-tositumomab needed to deliver a prescribed total-body dose of radiation (patient-specific therapeutic dose) (5). For the therapeutic administration, most patients received an infusion of 450 mg of unlabeled tositumomab followed by an infusion of 35 mg of 131I-tositumomab with the patient-specific activity of 131I to deliver the specified total-body radiation dose as described (5). Total-body doses ranged from 25 to 85 cGy in the phase I/II study (2). In all other studies, patients received 75 cGy unless the dose was reduced to 65 cGy because of decreased platelet counts (3). Immediately after the therapeutic administration, patient dose rates at 1 m were measured.
Patient Release Methodology
An algorithm for determining patient release after tositumomab and 131I-tositumomab therapy was developed on the basis of a retrospective analysis of patient data. Patients were eligible for release after completing a 3-step patient-release protocol. First, a patient interview must be conducted to evaluate the patient’s living and working conditions to determine whether the patient is able to care for himself or herself and whether the patient’s living or working arrangements are such that he or she can maintain a safe distance from family members and coworkers. Second, the patient-specific dose calculation, based on patient-specific total-body residence time (measured after the dosimetric administration) and the dose rate at 1 m (measured after the therapeutic administration), must be performed. For this analysis, values were standardized to the maximum tolerated total-body dose of 75 cGy. Thus, releasability for all patients was determined as if they had received a 75-cGy total-body dose.
Some common lifestyle behaviors, such as sleeping in a common bed or riding in a car, may increase the exposure of individuals to 131I. Therefore, the final step in the release process must involve the generation of written instructions regarding radiation safety precautions to maintain doses to other individuals of <5 mSv and as low as reasonably achievable (6). The instructions should specify how long these precautions must be followed. Therefore, the times necessary to follow these instructions were determined for each patient. Before patient release, the physician must have a high degree of confidence that the instructions will be followed. For released patients, the instructions were discussed before release and were given in writing to the patient.
Patient-Specific Dose Calculation to Determine Patient Releasability
Before patient release from the nuclear medicine facility, a patient-specific dose calculation must be performed to ensure that the maximally exposed individual will receive a radiation dose of <5 mSv. The dose calculation was based on the measured total-body residence time (τTB) and dose rate at 1 m ([mSv/h]1m ([mrem/h]1m)), obtained for each patient as per NRC guidance in Appendix B of Regulatory Guide 8.39 (7). The calculation also requires inclusion of a term to account for the fact that during the initial hours (approximately 3 h) after administration of the radiolabeled material, the patient may not void and the activity is therefore not removed from the body. Failure to include this component would underestimate the dose to total decay (9). The following equation was used for the patient-specific dose calculation for 131I-tositumomab:
 Eq. 1
where D∞ = total dose (mSv) from exposure to γ-radiation; Γ = exposure (dose) rate constant at 1 m (5.95 × 10−5 mSv m2/MBq h [0.22 mrem m2/mCi h] for 131I); Q0 = administered activity (MBq); τNV = residence time (h) of the nonvoided component (i.e., 1.443 times the physical half-life of the radionuclide). For 131I, with a physical half-life of 8.04 d, τNV = 278.4 h; and τTB = total-body residence time (h) of 131I-tositumomab.
Eq. 1
where D∞ = total dose (mSv) from exposure to γ-radiation; Γ = exposure (dose) rate constant at 1 m (5.95 × 10−5 mSv m2/MBq h [0.22 mrem m2/mCi h] for 131I); Q0 = administered activity (MBq); τNV = residence time (h) of the nonvoided component (i.e., 1.443 times the physical half-life of the radionuclide). For 131I, with a physical half-life of 8.04 d, τNV = 278.4 h; and τTB = total-body residence time (h) of 131I-tositumomab.
The total-body residence time, τTB, was determined from the total-body time–activity curve obtained during the dosimetric study. This curve may be multiexponential and therefore may have multiple components, such as the sum of the thyroidal and extrathyroidal components for Na131I as presented in Regulatory Guide 8.39 (7). The time–activity curve was determined in this study to be monoexponential by multiple total-body gamma-camera images of the patient after the dosimetric administration; therefore, Equation 1 was derived on the basis of the observed monoexponential clearance of 131I-tositumomab from the body. This equation can also be applied to any radionuclide that exhibits monoexponential clearance from the body when the appropriate physical half-life is used for the nonvoided component. (If a radiopharmaceutical exhibits multiexponential clearance from the body, Equation 1 is not applicable.)
Equation 1 uses an initial nonvoiding period of 3 h (9) and an occupancy factor for this component of 0.75; at other times, an occupancy factor of 0.25 is used. This equation can be adapted for nonvoiding intervals other than 3 h by replacing the number 3 in Equation 1 with the appropriate value for the specific radionuclide. Finally, this equation includes the additional conservative assumption that 100% of the 131I activity does not leave the body during the initial nonvoiding period instead of the 80% value used in Regulatory Guide 8.39 (7).
Substituting the value for the residence time of the nonvoided component of 278.4 h for 131I, Equation 1 can be simplified to:
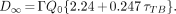 Eq. 2
Eq. 2
Substituting the theoretic dose rate (i.e., the dose rate in air from a point source of activity, Q0) in mSv/h at 1 m, ΓQ0, with the measured dose rate at 1 m (mSv/h)1m, Equation 2 becomes:
 Eq. 3
Equation 3 was used to estimate the maximum potential dose (TEDE) to individuals exposed to the patient. Equation 3 can also be rearranged to calculate the maximum dose rate at 1 m that will result in a dose of <5 mSv to individuals exposed to the patient:
Eq. 3
Equation 3 was used to estimate the maximum potential dose (TEDE) to individuals exposed to the patient. Equation 3 can also be rearranged to calculate the maximum dose rate at 1 m that will result in a dose of <5 mSv to individuals exposed to the patient:
 Eq. 4
Eq. 4
Equation 4 is the case-specific calculation for patient release, which accounts for the patient occupancy factor and the nonvoiding time period. If the patient’s dose rate at 1 m measured immediately after the therapeutic administration was <5/(2.24 + 0.247 τTB), then the patient was releasable. The releasable dose rate is predicated on an occupancy factor of 0.25 at a distance of 1 m and limits the maximal exposure to <5 mSv. Some lifestyle behaviors, such as sleeping in a common bed or extensive traveling with a partner, may result in a higher dose rate or a closer distance. In addition, a patient should limit his or her exposure to children, infants, or pregnant women to <1 mSv. Therefore, released patients were provided written instructions to maintain doses to other individuals of <5 mSv according to the new NRC regulations.
A look-up table has been generated to easily implement the determination of patient releasability based on Equation 4 (Table 1). Releasable dose rates at 1 m, as a function of patient residence time, are listed in Table 1 (an abbreviated look-up table adapted from the physicians’ guidelines (15)). These dose rates represent the maximum allowable for a specific residence time to ensure that no individual will likely receive a dose of ≥5 mSv. A patient is immediately releasable if his or her measured dose rate at 1 m is less than the value given in Table 1 for the patient-specific residence time. This table was generated on the basis of 2 patient-specific factors, residence time and measured dose rate at 1 m, which must be included in the patient’s record at the time of release. Table 1 assumes an occupancy factor of 0.25 and the values in the table must, therefore, be adjusted for occupancy factors other than 0.25. For example, if there is justification for using a lower occupancy factor of 0.125, the values of the dose rates in the table should be doubled. If a higher occupancy factor of 0.5 is necessary, the values in the table should be halved.
Determination of 131I-Tositumomab–Treated Patient Releasability According to Patient Dose Rate at 1 Meter and Total-Body Residence Time
The predicted TEDE to the maximally exposed individual was calculated on the basis of the individualized measured parameters of total-body residence time and dose rate using Equation 3.
Patient Instructions Before Release
The patient must limit the time he or she is in contact with other individuals; however, the NRC provides only general guidance for these precautions. The patient’s measured total-body residence time and dose rate can also be used to determine the times necessary for patient instructions to remain in effect to limit certain activities. Therefore, before release, patients also received patient-specific instructions to limit the radiation exposure to family members and caregivers by limiting the time that they are in close contact on the basis of their own residence time and dose rate. The calculations for determining the amount of time necessary for instructions to remain in effect to minimize the radiation dose to sleeping partners, traveling companions, children and pregnant women, and members of the general public are given below.
Dose to Sleeping Partner.
Assumption: Partner receives exposure for 4.5 h/d at 1 m before resumption of normal sleeping. Normal sleeping is 6 h each day at a distance of 0.3 m.
 Eq. 5
where R0 = the initial dose rate (mSv/h) at 1 m; t = the total-body residence time (h); and D∞ = the dose (mSv) to the maximally exposed individual at the time of patient release.
Eq. 5
where R0 = the initial dose rate (mSv/h) at 1 m; t = the total-body residence time (h); and D∞ = the dose (mSv) to the maximally exposed individual at the time of patient release.
For the TEDE to be <5 mSv, the time required before sleeping 6 h/d at 0.3 m, tsleep (d) must satisfy:
 Eq. 6
No limitations for D∞ < 0.674 mSv
Eq. 6
No limitations for D∞ < 0.674 mSv
Dose to Adult on 4-Hour Trip.
Assumption: Passengers receive no dose before and after trip. Distance between patient and other passengers is assumed to be 0.3 m.
 Eq. 7
Eq. 7
Selecting τ = 60 h sets an upper limit = 1.72 D∞ e−ttrip/τ. For the TEDE to be <5 mSv, ttrip (d) must satisfy:
 Eq. 8
No limitations for D∞ < 2.907 mSv
Eq. 8
No limitations for D∞ < 2.907 mSv
Dose to Children and Pregnant Women
Assumption: Infants or young children and pregnant women receive no dose before the period without limitations. Normal contact is assumed to be 30 min/d of direct contact at a distance of 0.1 m and 6 h of exposure per day at a distance of 1 m.
 Eq. 9
Eq. 9
For the TEDE to be <1 mSv, the time required before normal contact, tinfant (d), must satisfy:
 Eq. 10
No limitations for D∞ < 0.374 mSv
Eq. 10
No limitations for D∞ < 0.374 mSv
Dose to Members of General Public
Assumption: Members of the general public receive no dose before the period without limitations. Normal exposure for 6 h/d (i.e., an occupancy factor of 0.25) at 1 m is assumed.
 Eq. 11
Eq. 11
For the TEDE to be <1 mSv, the time required before 6 h of exposure per day at 1 m, tpublic (d), must satisfy:
 Eq. 12
No limitations for D∞ ≤ 1 mSv
Eq. 12
No limitations for D∞ ≤ 1 mSv
Equations 5–12 implicitly assume monoexponential clearance of 131I-tositumomab from the body. (As was the case for the patient-specific dose calculation, these equations are not applicable for radiopharmaceuticals that exhibit multiexponential clearance from the body.) Further, it is assumed in these equations that the dose rates from the patients at distances, d, other than the measurement distance of 1 m (i.e., 0.1 and 0.3 m) can be approximated by 2 d−1. This approximation was based on actual dose rate measurements obtained at 0.3 and 1 m in 29 131I-tositumomab patients, where it was observed that the mean 0.3 m:1 m dose rate ratio was 3.1 ± 1.2 (range, 1.4–6.7) (10). Thus, the 2-d−1 approximation is conservative. The use of the 2-d−1 approximation is further validated by a theoretic calculation under the assumption that the patient simulates a line (rather than a point) source of activity (9,16). In this case, the dose rate is inversely proportional to the distance and directly proportional to the angle, in radians, subtended by the line source; the results of these calculations indicate that 2 d−1 is an accurate approximation for the expected dose rates at 0.3 and 0.1 m. It may be more accurate to simply perform additional dose rate measurements at 0.1 and 0.3 m, as others have suggested (10,17), but we believe that the use of a conservative approximation minimizes the need for these additional measurements, while still maintaining accuracy, thus making the method more practical.
The predicted total effective dose equivalent (D∞), as determined by Equation 3, is compared with the values in Table 2 (15) (use the column that is greater than or equal to the calculated value for D∞). To determine the times necessary before sharing a bed, taking long trips, interacting with children and pregnant women, and returning to work (Table 2, instructions 1–4) (15), the value in the appropriate column is multiplied by the patient’s residence time (h) to obtain the duration (d) for each instruction to the released patient. For fractions, the number of days is rounded up to a full day. The durations for instruction numbers 1 and 2 are based on 5-mSv TEDE, whereas those for numbers 3 and 4 are based on 1-mSv TEDE.
Determination of Instruction Times Necessary to Limit Close Contact with Others
RESULTS
Patients
The pertinent demographics and disease characteristics of the patients (n = 139) receiving tositumomab and 131I-tositumomab therapy are listed in Table 3. Most patients (73%) had low-grade follicular non-Hodgkin’s lymphoma and 92% of the patients were classified as having stage III or stage IV disease. Patients received therapeutic administration of 131I-tositumomab ranging from 1,221 to 5,957 MBq (33–161 mCi), dependent on dosimetry studies, to deliver a total-body dose of 25–85 cGy. In the phase I/II study, the maximum tolerated total-body dose was established for future studies to be 75 cGy. The mean total-body residence time of 131I-tositumomab (±SD) was 93.5 ± 15.5 h and ranged from 50 to 129 h (Fig. 1). The coefficient of variation was 16.6%. The mean administered 131I activity (±SD) was 3,119 ± 1,066 MBq (84.3 ± 28.8 mCi) (range, 1,221–5,957 MBq [33–161 mCi]). The mean dose rate at 1 m (±SD), measured immediately after the therapeutic infusion, was 0.109 ± 0.032 mSv/h (10.9 ± 3.2 mrem/h (range, 0.04–0.24 mSv/h [4–24 mrem/h]) (Fig. 2).
Distribution of 157 total-body residence times for patients receiving 131I-tositumomab obtained from 3 to 8 total-body γ-counts obtained over 6–7 d during dosimetric study.
Distribution of 157 measured dose rates at 1 m obtained immediately after therapeutic administration of 131I- tositumomab (1,221–5,957 MBq).
Patient Demographics and Disease Characteristics
Theoretic Versus Measured Dose Rate
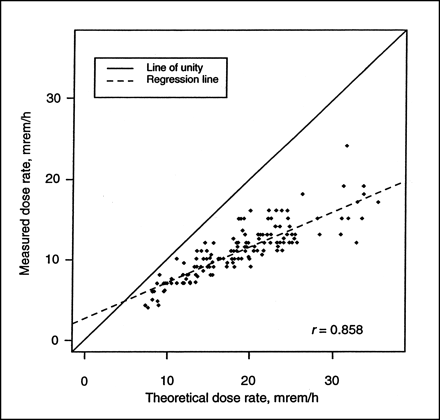
The theoretic and measured dose rates at 1 m immediately after infusion were compared. The theoretic dose rate at 1 m was calculated by multiplying the administered activity of 131I (MBq) by the known dose equivalent rate constant for 131I of 5.95 × 10−5 mSv/MBq h (0.22 mrem/mCi h) at 1 m. The correlation between the theoretic and the observed dose rate was good (r = 0.858). However, because the theoretic dose rate does not account for photon attenuation and nonpoint–source distribution of the 131I activity within the patient, it was consistently higher than the observed dose rate (Fig. 3). The mean observed dose rate (±SD) was 60% ± 10% of the theoretic dose rate (range, 37%–90%). Because the observed dose rates after administration of 131I-tositumomab were approximately 60% of the theoretic rates, the initial dose rate (mSv/h) at 1 m immediately after therapy is expected to be 3.57 × 10−5 (0.60 × 5.95 × 10−5) times the administered activity of 131I (MBq) (initial dose rate [mrem/h] at 1 m is 0.132 [0.60 × 0.22] times the administered activity [mCi]). Larger patients had a slightly lower measured dose rate and smaller patients had a slightly higher measured dose rate.
Comparison of measured and theoretic dose rate at 1 m after therapeutic administration of 131I-tositumomab (n = 157). Theoretic dose rate is calculated using dose equivalent rate per unit activity of 131I (5.95 × 10−5 mSv/MBq h [0.22 mrem/mCi h] at 1 m), which is intended for point source of radioactivity in air and not distributed source in patient. Correlation between 2 measures was 0.858, with measured dose rate, on average, 60% of theoretic dose rate.
The percentage difference between the theoretic and observed dose rates increased with higher administered activity (Fig. 3). The measured dose rate at 1 m per unit activity infused predictably decreased with increasing size, expressed as body mass (Fig. 4), or body surface area because of increasing photon attenuation.
Plot of measured dose rate per MBq (mCi) versus patient mass obtained at 1 m immediately after therapeutic dose of 131I-tositumomab. Measured dose rates per MBq (mCi) were below theoretic dose rate per mCi and tended to be lower in patients with greater mass (r = −0.547).
Radiation Doses to Maximally Exposed Individuals
The mean estimated doses to individuals maximally exposed to patients (i.e., TEDE) were calculated using Equation 3 on the basis of a 0.25 occupancy factor at 1 m. These estimates revealed a mean TEDE (±SD) of 3.06 ± 0.56 mSv (306 ± 56 mrem) (range, 1.95–4.96 mSv [195–496 mrem]) (Fig. 5). All patients were releasable on the basis of the predicted dose to the maximally exposed individuals.
Distribution of 157 maximum predicted doses to individuals exposed to patients receiving tositumomab and 131I-tositumomab therapy after immediate release following therapeutic administration. Maximum doses are based on occupancy factor of 0.25 at 1 m.
The mean recommended times to limit close contact with others were calculated for the entire patient population on the basis of the total-body residence times and measured dose rates (Table 4). The mean time to avoid sleeping in a common bed for the entire patient population was 7.8 d (range, 2.8–14.6 d). The mean times to avoid close travel for 4 h, daily contact with infants or pregnant women, and daily close contact of >6 h with individuals other than the caregiver or family member were 0.3, 8.1, and 4.3 d, respectively.
Recommended Times to Avoid Close Contact with Others After Tositumomab and 131I-Tositumomab Therapy
The following is a practical example of the generation of the instructions in Table 4 using the values listed in Table 2. After a 185-MBq (5 mCi) dosimetric administration of 131I-tositumomab, the residence time for a patient was determined to be 73 h. Immediately after the therapeutic administration of 131I-tositumomab, the dose rate at 1 m from the patient was 0.18 mSv/h. Using Equation 3, the TEDE, D∞ = 0.18 mSv/h × (2.24 + 0.247 × 73 h), was determined to be 3.65 mSv. Therefore, the 3.75-mSv column in Table 2 was used to calculate the duration for each instruction. The times necessary for the instructions to remain in effect for this patient would be as follows:
Sleep in a separate bed (at least 2-m separation) = 73 × 0.102 = 8 d
Do not take a long trip (≥4 h) sitting near others (e.g., car, train, airplane, bus) = 73 × 0.011 = 1 d
Stay at least 2 m from children and pregnant women = 73 × 0.096 = 7 d; if no direct contact with children or pregnant women = 73 × 0.055 = 4 d
A physician may want to limit the dose to infants or young children and pregnant women to 1 mSv. Normal contact (after the avoidance period) with children and pregnant women is assumed to be 30 min/d of direct contact and 6 h/d at 1 m. In this case, the instruction duration is obtained from Table 2, row 3. If no direct contact is planned, the instruction duration is obtained from Table 2, row 4.
Minimize time spent in close contact with others and delay return to work = 73 × 0.055 = 4 d
A physician may also want to limit the dose to members of the general public to 1 mSv (and apply the 5-mSv limit only to adult, nonpregnant family members or the maximally exposed individual). In this case, the instruction duration is obtained from Table 2, row 4.
DISCUSSION
This study showed that after 157 therapeutic administrations of 131I-tositumomab all patients were immediately releasable from the hospital in accordance with the current NRC guidelines. The mean estimated radiation dose for maximally exposed individuals was 3.06 mSv, well within the NRC limit of 5 mSv. These findings were verified by a recent study in which the radiation exposure of family members was measured directly by dosimeter readings (11,18). In this study, the actual measured doses ranged from 0.1 to 3.54 mSv (mean, 1.44 mSv). When the dosimetry readings were adjusted for infinite time of exposure, the range was 0.27–4.51 mSv, with a mean measured dose of 1.68 mSv. In our study the calculated maximum TEDE ranged from 1.95 to 4.96 mSv. These 2 studies, interpreted together with other outpatient radioimmunotherapy studies (10,19), and measurements obtained on family members of thyroid cancer or hyperthyroid patients (13,14,20,21) confirm that patients treated with 131I-tositumomab are releasable and will not expose individuals to radiation doses of >5 mSv.
Because releasability is determined only by dose rate at 1 m and the patient’s total-body residence time, a comparison of these patient-specific parameters to predefined values within a look-up table simplifies the process of determining patient releasability. Furthermore, patient-specific calculations can be performed on the basis of these simple variables to develop instructions that include the necessary times that a patient is required to limit close contact with others. These improvements in patient-specific calculations and guidelines for patient release provide the basis for the development of standardized patient-release methodologies. Such methodologies will facilitate use of radioimmunotherapy in the community hospital setting, thereby increasing the availability of outpatient radioimmunotherapy. Additionally, the expanded access of outpatient radioimmunotherapy through the implementation of this methodology will shorten hospital stays, lower health care costs, and reduce radiation exposure of hospital personnel.
Once it has been determined that the patient cannot only be released (by patient-specific dose calculation), but is also releasable (by patient interview), the released patient must be provided with written instructions to maintain doses to other individuals of <5 mSv and as low as reasonably achievable (6). The instructions and all related discussions must be presented in a simple and clear manner so that the patient understands the importance of following them. A copy of the instructions must be provided to the patient and a copy must be maintained on file. The Society of Nuclear Medicine published a pamphlet in 1987 that provides information and instructions for patients receiving treatment with orally administered radioiodine. Although this pamphlet was written for the release of patients containing an activity of 131I of <1,110 MBq, the NRC considers the instructions in this pamphlet to be an acceptable method for meeting the requirements of 10 CFR 35.75(b) (6) provided the avoidance times are appropriate for the activity and the medical condition. These same instructions can be given to patients released after tositumomab and 131I-tositumomab therapy and should be followed for a period of time dependent on the patient-specific total-body residence time and dose rate. The instructions used in this study ensure that the TEDE to maximally exposed individuals is <5 mSv. Because the 131I is given intravenously and is attached to the antibody, most of the potential contamination problems present with orally administered Na131I will not be a problem.
When measuring the dose rate at 1 m to determine patient releasability, it is essential that an appropriate survey meter be used. Calibrated ionization chambers should be used for all dose rate measurements of patients who have received radioimmunotherapy. Calibrated ion chambers are energy independent and yield accurate dose rate values regardless of the radionuclide being measured. Before measuring a patient’s dose rate, one should calculate the expected dose rate (i.e., on average 0.132 times the administered millicuries for 131I tositumomab). Any large deviations from the expected value may suggest a calibration problem or an incorrect measuring distance.
It must be emphasized that the dose estimates in this study are conservative and the expected exposure to others will be less than those calculated. The dose calculations based on measured dose rate at 1 m and patient-specific factors will significantly overestimate the actual dose to others from patients released after 131I therapy (22). The primary reason for this is that the patient dose rate is a measurement of surface entrance dose rate, not whole-body dose rate. Measured dose rates are conservative by a factor of 2 because they represent surface dose to an individual with no correction for attenuation by the body of the exposed individual. In addition, the dose estimates may be overestimated because of the use of a conservative occupancy factor.
Because each radionuclide has its own half-life and emission spectrum, the dose-rate–based patient release criteria developed in this study potentially can be applied to the release protocols for patients treated with any radionuclide. Patients were released by comparing the measured dose rate at 1 m with the calculated maximum releasable dose rate at 1 m based on the patient’s total-body residence time, as determined from a dosimetry study. All tositumomab- and 131I-tositumomab-treated patients in this study were determined to be safe for release because the estimated dose to the maximally exposed individual was <5 mSv.
CONCLUSION
A simple method for patient release based on the patient-specific total-body residence time and dose rate after tositumomab and 131I-tositumomab therapy was developed. All patients were found to be immediately releasable; however, patients need appropriate evaluation and instructions from their physicians before a decision to perform outpatient therapy is made. A methodology to determine the times necessary to avoid certain activities to limit exposure to other individuals was developed to provide written instructions to each patient. Consistent with the recently revised regulations governing patient release, it should be possible to perform many radionuclide therapy procedures on an outpatient basis. Use of this methodology for patient release can safely expand the use of outpatient radionuclide therapy to the community hospital setting. The result of expanded outpatient radioimmunotherapy is expected to result in increased patient quality of life, reduced hospital costs, and a reduction in radiation exposure to hospital personnel.
Footnotes
Received May 14, 2001; revision accepted Sep. 25, 2001.
For correspondence or reprints contact: Jeffry A. Siegel, PhD, Nuclear Physics Enterprises, 216 Society Hill, Cherry Hill, NJ 08003.
Email: siegelja{at}aol.com