Abstract
Radiomicrosphere treatment involves the intrahepatic arterial administration of 90Y-resin or 90Y-glass microspheres. The microspheres are biocompatible, but not biodegradable, and little to no 90Y leaches from the microspheres. Without any bioelimination, the β-dose delivery is generally confined to the liver. Although U.S. Nuclear Regulatory Commission requirements permit patients treated with these microspheres to be released without the need for dose determination or patient instructions, there are important radiation safety issues that need scientific clarification. We carefully evaluated the radiation exposure mechanisms, including the bremsstrahlung radiation doses to others, for a variety of lifestyle behaviors. Dose estimates were also made for several practical and theoretic situations involving the patient's gonads, an embryo or fetus, and a nursing infant. For the infant, we evaluated the potential β-dose that might be introduced via breast milk ingestion. The bremsstrahlung component of the decay scheme of the pure β-emitter 90Y has traditionally been ignored in internal and external dose calculations. Because the production of in vivo bremsstrahlung with the high-energy pure β-particle–emitting radionuclides used for therapeutic purposes is sufficient to permit external detection and imaging, we believe that the contribution of such radiation should be considered with regard to patient release; we therefore chose to evaluate this potential external radiation hazard. In all cases, the estimated doses were very small, indicating that no patient restrictions are required for radiation safety purposes after the release of a patient who has been treated with 90Y-microspheres.
The radiomicrosphere treatment concept was introduced during the 1960s, and the techniques and clinical applications have been extensively described in the literature (1–5). The delivery of radiation treatment via intrahepatic arterial administration of 90Y-microspheres is commonly referred to as selective internal radiation treatment. 90Y is a high-energy pure β-particle–emitting radioisotope that is incorporated in biocompatible microspheres. The radiolabeled microspheres preferentially localize in tumor arterial vasculature, resulting in very high radiation doses to tumors while maintaining radiation doses to normal hepatic parenchyma that are within tolerable limits.
90Y-microsphere treatment is a true interdisciplinary treatment modality. Treatment planning and execution involve interventional radiology, radiation oncology, and nuclear medicine. The technique is referred to as radioembolization by interventional radiologists because of its technical similarities to chemoembolization. In reality, however, both the theory and the practice of 90Y-microsphere treatment are different from those of chemoembolization. Unlike chemoembolization, 90Y-microsphere treatment requires optimal perfusion and blood flow to enhance free radical–dependent cell death. Radiation in combination with embolization-induced hypoxia is therefore undesirable. The biologic response is optimized with the preservation of flow to the target area and hence oxygenation. Brachytherapy (or microbrachytherapy), a term used by radiation oncologists, originates from the approval of 90Y microspheres by the U.S. Food and Drug Administration (FDA) as medical devices. The actual use of 90Y-microspheres, however, is as a radiopharmaceutical. The material is prepared in a solution and may be assayed in a dose calibrator. The prescription is in units of activity, and the absorbed dose to the tumor target is not used in the treatment prescription. Prescribed activity can be adjusted by volumetric manipulation. The agent is administered by use of a syringe or injector via a catheter into an artery. We prefer to refer to the technique as radiomicrosphere treatment, as that phrase best describes the essence of the treatment and may help avoid potential confusion in the oncology community and other interdisciplinary conflicts.
The purpose of this article is to focus on radiation safety considerations after the treatment of patients with 90Y-microspheres. Radiation-absorbed doses to radiation-sensitive tissues of the patient and likely exposures of others because of the bremsstrahlung radiation component (and the β-component via breast milk ingestion by a nursing infant) of 90Y are carefully evaluated. The dose estimates can be used to enable treating physicians to provide answers to commonly asked questions and to provide more informed advice to staff, treated patients, and their caregivers or family members.
RADIOPHARMACEUTICALS FOR 90Y-MICROSPHERE TREATMENT
Two commercial products are available in the United States at the present time. SIR-Spheres (Sirtex Medical) resin microspheres are polymer beads designed to be between 20 and 60 μm in diameter and loaded with 90Y at a specific activity of 40–70 Bq per sphere (6,7). TheraSphere (MDS Nordion) glass microspheres measure between 20 and 30 μm and are loaded with 90Y at a specific activity of 2,400–2,700 Bq per sphere (8). Typical treatment activities are in the range of 2–6 GBq; the maximum administered activity for resin microspheres has generally been limited to 3 GBq, whereas it may be as high as 9 GBq for glass microspheres. 90Y-glass microspheres are approved by the FDA for the treatment of nonresectable hepatocellular carcinoma under the provisions of a humanitarian device exemption. 90Y-resin microspheres have received FDA premarket approval for concurrent use with fluorodeoxyuridine for the treatment of hepatic metastases from colorectal cancer.
The manufacturing of resin microspheres is essentially different from that of glass microspheres. 90Y-resin microspheres are constructed chemically by incorporating 90Y on the surface of the resin matrix, whereas 90Y-glass microspheres are produced by neutron bombardment of an yttrium oxide–bearing substrate that is integrally bound within the glass matrix of the microsphere in a high-flux reactor.
There are 2 main methods for producing 90Y for radiolabeling of resin microspheres: nuclear reactor production and 90Sr/90Y generator production. The generator produces carrier-free 90Y; the only significant potential radionuclide impurity is 90Sr. Reactor-produced 90Y will contain several parts per million of radioactive impurities, the most significant being 88Y, which decays by electron capture or positron emission with a physical half-life (t1/2) of 106.6 d. By virtue of the generation process, long-lived radiocontaminants, such as 152Eu, may be present in 90Y-glass microspheres. The U.S. Nuclear Regulatory Commission (NRC) recently issued an Information Notice to alert licensees to the presence of radioactive contaminants in glass and resin microspheres and to possible problems with their disposal in accordance with 10 CFR 35.92, pertaining to decay in storage (9). The radiocontaminant profiles of resin and glass microspheres show differences related to their production mechanisms. Resin microspheres have been shown to contain detectable amounts of 88Y, and glass microspheres may have 88Y, 154Eu (t1/2 = 8.8 y), 152Eu (t1/2 = 136 y), 57Co (t1/2 = 270.9 d), and 60Co (t1/2 = 5.27 y). Dose calculations have indicated that the radiocontaminant dose does not exceed the medical event limit, but licensees may need to be concerned about disposal of the microspheres because of the long-lived contaminants that may be present and detectable long after all of the 90Y has decayed.
Glass microspheres are not known to have any free 90Y in the treatment vial, nor does any significant amount of 90Y leach from the spheres (10). Resin microspheres, on the other hand, may have trace amounts of free 90Y on their surface, perhaps as high as 0.4% of the 90Y administered activity, which can be excreted in the urine during the first 24 h. Trace amounts (25–50 kBq/L/GBq) of urinary excretion are a possibility in the first 24 h after implantation (7).
RADIATION SAFETY CONSIDERATIONS AFTER 90Y-MICROSPHERE TREATMENT
In the United States, 90Y-microsphere therapy is regulated by the NRC, pursuant to 10 CFR 35.1000, and patient release must follow the requirements in 10 CFR 35.75 (11). A licensee may release patients, regardless of administered activity, if it can be demonstrated that the total effective dose equivalent (TEDE) to another individual from exposure to a released patient is not likely to exceed 5 mSv (0.5 rem). In addition, pursuant to 10 CFR 35.75(b), licensees must provide a released patient with written instructions on actions recommended to maintain doses to other individuals as low as reasonably achievable if the dose to any other individual is likely to exceed 1 mSv.
Estimated Radiation Dose to Maximally Exposed Individual
The bremsstrahlung radiation component is considered here because the involved β-dose would be negligible. The following equation can be used to estimate the dose that an individual is likely to receive from exposure to a released patient (12):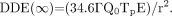 Eq. 1In this equation, DDE (∞) is external exposure, attributable to bremsstrahlung radiation, to total decay (i.e., out to infinite time) in millisieverts (millirems); Γ is the specific bremsstrahlung constant for 90Y in soft tissue (1.52 × 10−3 mSv/cm2/MBq/h) (13); Q0 is the administered activity in megabecquerels; Tp is the physical t1/2 of the radionuclide in days (2.67 d for 90Y); E is the occupancy factor at 1 m (0.25); and r is distance from the patient (1 m [100 cm]).
Eq. 1In this equation, DDE (∞) is external exposure, attributable to bremsstrahlung radiation, to total decay (i.e., out to infinite time) in millisieverts (millirems); Γ is the specific bremsstrahlung constant for 90Y in soft tissue (1.52 × 10−3 mSv/cm2/MBq/h) (13); Q0 is the administered activity in megabecquerels; Tp is the physical t1/2 of the radionuclide in days (2.67 d for 90Y); E is the occupancy factor at 1 m (0.25); and r is distance from the patient (1 m [100 cm]).
Equation 1 represents the dose to a putative individual likely to receive the highest dose attributable to the bremsstrahlung radiation component from exposure to released 90Y-treated patients. It is also a conservative dose estimation because it represents a point-source model and does not take into account the considerable attenuation of bremsstrahlung photons by the body (14). The equation was derived under the assumptions of instantaneous activity uptake, uniform activity deposition, and no biologic elimination. Equation 1 can be solved for the maximum allowable administered activity for authorizing patient release on the basis of the 5-mSv regulatory dose limit; this value has been reported to be 1,420 GBq (13). In compliance with the dose limit in 10 CFR 35.75(a), licensees may release patients from their control if the activity administered is no greater than 1,420 GBq. If release is based on administered activity, no record is required. Patient instructions are required only if the dose to other individuals is likely to exceed 1 mSv (0.1 rem). This dose would correspond to one fifth of the maximum allowable release value, corresponding to an administered activity of 284 GBq. Because all patients treated with 90Y-microspheres receive a treatment activity that is significantly lower than this value, all patients can be released; no records or instructions are required by the NRC.
It should be noted that the specific bremsstrahlung constant is a gross approximation, because it was derived by use of the mean energy rather than the actual bremsstrahlung energy spectrum for 90Y (13). It is nevertheless a useful calculation construct, because the long “tail” of the bremsstrahlung energy spectrum represents a very small proportion of the total radiative energy losses in vivo.
A patient who has been treated with 90Y-microspheres will generally have an activity distribution in the body that is confined to the liver (in some cases, there may also be some shunting of activity to the lungs). Use of the point-source model (i.e., Eq. 1) for patients with such extended activity distributions is reasonable at distances of 1 m or greater but has been shown to overestimate the radiation-absorbed dose to exposed individuals interacting at closer distances, sometimes by a very significant amount (15). Doses of 18.8, 9.2, and 1.5 μSv/h at 0.25, 0.5, and 1 m, respectively, have been reported at approximately 6 h after the administration of a 2.1-GBq average activity of 90Y-microspheres (7). The measured values at distances of 0.25 and 0.5 m from the patient indicate that the inverse square approximation is not valid at close distances, as expected, because the activity is generally distributed in the liver and resembles a line more than a point source of activity. For example, because it is common practice to measure a dose at 1 m and then use the inverse square dose–distance approximation, as given in Equation 1, to estimate the dose at 0.25 m, the predicted dose at this distance would have been a factor of 16 times higher than that measured at 1 m, resulting in a 28% overestimation of the value relative to the measured value. On the basis of these measured data, doses at distances closer than 1 m may be approximated from the 1-m dose measurement by multiplication by the factor 3/d, where d is the distance of interest in meters. This relationship will be used in all subsequent dose calculations involving distances closer than 1 m, according to the following equation: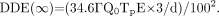 Eq. 2It is important to note that the NRC, in its guidance document governing the release of patients given radionuclide therapy, does not determine activity limits for 90Y and other β-emitters “because of the minimal exposures to members of the public resulting from activities normally administered for diagnostic or therapeutic purposes” (12). The NRC guidance document does not even list an exposure rate constant for 90Y because, as stated, the activity is not based on β-emissions. Because of the assumed minimal risk of exposure of other individuals, the NRC does not require the determination of activity or dose limits or the issuance of instructions before patients treated with pure β-emitting radionuclides can be released. This guideline may be challenged because it ignores the bremsstrahlung component of the decay scheme of 90Y in the determination of the external radiation dose.
Eq. 2It is important to note that the NRC, in its guidance document governing the release of patients given radionuclide therapy, does not determine activity limits for 90Y and other β-emitters “because of the minimal exposures to members of the public resulting from activities normally administered for diagnostic or therapeutic purposes” (12). The NRC guidance document does not even list an exposure rate constant for 90Y because, as stated, the activity is not based on β-emissions. Because of the assumed minimal risk of exposure of other individuals, the NRC does not require the determination of activity or dose limits or the issuance of instructions before patients treated with pure β-emitting radionuclides can be released. This guideline may be challenged because it ignores the bremsstrahlung component of the decay scheme of 90Y in the determination of the external radiation dose.
We investigated various exposure scenarios to assess whether the NRC implicit assumption that there is no significant external radiation hazard from internally emitted β-rays is reasonable and appropriate. Even though the β-dose is very small and the bremsstrahlung component is also admittedly minimal, the bremsstrahlung dose to other individuals may not always be negligible, especially if large amounts of 90Y are involved and interaction distances between patients and other individuals are small. In such cases, the resulting radiation hazard may be of concern and should at least be systematically evaluated, because it may be prudent in such cases to issue instructions to released 90Y-microsphere–treated patients.
Estimated Radiation Dose to Others Likely to Be Exposed
The estimated total dose to individuals who may be in close contact with a released 90Y-microsphere–treated patient (e.g., infant, sleeping partner, or extensive traveling companion) may be of concern. Given that there is a very real potential for others to interact at distances closer than 1 m from the patient (16), dose estimates based on various lifestyle behaviors are calculated later.
Dose to Household Member.
If it is conservatively assumed that the interaction distance is 1 m from a patient who has received 3 GBq of 90Y-microspheres for 6 h/d (E = 0.25) to total decay (assumptions made for Eq. 1), then
Dose to Caregiver, Sleeping Partner, or Extensive Traveling Companion.
If it is conservatively assumed that the interaction distance is 0.3 m from a patient who has received 3 GBq of 90Y-microspheres for 6 h/d (E = 0.25) to total decay, then If the patient needs significant care, then a conservative assumption would be the use of an occupancy factor of 0.5 (i.e., the caregiver spends 50% of the day at a distance of 0.3 m from the patient), and
If the patient needs significant care, then a conservative assumption would be the use of an occupancy factor of 0.5 (i.e., the caregiver spends 50% of the day at a distance of 0.3 m from the patient), and
Dose to Infant, Child, or Pregnant Woman.
If it is conservatively assumed that the interaction distance is 0.1 m from a patient who has received 3 GBq of 90Y-microspheres for 1 h/d (E = 0.042) to total decay, then
These dose estimates indicate that a 1-mSv dose limit is not likely to be reached with administered activities of 90Y-microspheres of less than 14.3 GBq. Thus, even for individuals who potentially could spend considerable time at close distances, their exposures are still not likely to approach the 1-mSv dose limit set by the NRC for providing instructions to released 90Y-microsphere–treated patients, let alone the NRC TEDE limit of 5 mSv (11). This is true even for glass microsphere–treated patients, who may receive administered activities of up to 9 GBq, at which the doses would be a factor of 3 higher than those calculated earlier. These dose estimates should be of value to treating physicians and radiation safety personnel, for example, in better addressing the concerns of a pregnant caregiver, such as a nurse, who may be concerned about interacting with a patient who has received radiomicrosphere treatment.
In a study that measured the radiation doses to family members of patients treated with 90Y-ibritumomab tiuxetan, a treatment for non-Hodgkin's lymphoma, it was determined that the radiation doses ranged from 0.014 to 0.079 mSv over the 7-d monitoring period; these doses are in the range of background radiation (17). The patients had unrestricted contact with family members; the only recommendations to the family were to avoid contamination from any body fluids (e.g., saliva, blood, urine, or stool). These patients, who can receive a maximum administered activity of 1,184 MBq, can be immediately released without regard to activity administration, because no individual is likely to receive an exposure that would even remotely approach, let alone exceed, the regulatory dose limit of 5 mSv. It should be noted that 90Y-ibritumomab tiuxetan activity is more widely dispersed in the body than that of 90Y-microspheres; therefore, for equivalent administered activities, the doses to others at close distances would theoretically be somewhat lower (15).
Microsphere-treated patients represent an even lower radiation safety risk for individuals potentially exposed to these patients because there is no biologic elimination of 90Y, as it remains fixed in the liver, tumors, or lungs, with essentially no radioactivity appearing in any body fluid. As stated previously, glass microspheres are not known to be present in any body fluid, whereas trace amounts of 90Y activity may be excreted in the urine of resin microsphere–treated patients for the first 24 h.
Estimated Radiation Dose to Nursing Infant
The issue of breast milk excretion of radiopharmaceuticals and ingestion of the associated radionuclides by a nursing infant is a major radiation safety concern. Certainly, radiomicrosphere treatment should not be administered to a breast-feeding patient; however, we believe that a reasonable estimation of the radiation dose to a nursing infant is still rather instructive. Pursuant to 10 CFR 35.75(b), if the TEDE to a nursing infant or child could exceed 1 mSv, with the assumption of no interruption of breast-feeding, then instructions that include guidance on the interruption or discontinuation of breast-feeding and information on the potential consequences, if any, of failure to follow this guidance must also be provided to a released patient. For most radionuclides, the interruption of breast-feeding is not thought to be necessary, and the NRC makes no mention of or recommendation for such an interruption in patients receiving 90Y (12).
There are 2 potential radiation hazards for an infant. The first hazard is that ingestion of contaminated breast milk may result in a significant internal source of radiation doses to some of the organs of the infant. Only free 90Y, if present, can potentially reach the systemic circulation of the treated mother. As stated previously, glass microspheres are not of any concern in this respect. Resin microspheres, on the other hand, may have trace amounts of free 90Y on their surface, perhaps as high as 0.4% of the 90Y administered activity, but amounting to no more than 12 MBq (0.4% of a maximal 3-GBq administration) in the mother's bloodstream for the first 24 h. Of this amount, only a small fraction can potentially reach the breast milk and be ingested by the nursing infant. Furthermore, any activity ingested by the nursing infant will be poorly absorbed from the gastrointestinal (GI) tract (only approximately 0.01% of the activity in the GI tract will be absorbed into the blood), and the internal β-dose to the nursing infant will be confined to the GI tract. The exact mechanism and amounts of free 90Y accretion in breast milk are unclear because detailed kinetic studies have not been performed. Most of the free 90Y will remain in the liver; only trace amounts have been detected in the urine, and no contamination has been detected in any other body fluids or secretions (7). The following method is a conservative and reasonable approach for estimating the internal dose potentially involved.
First, assume that a nursing baby ingests 1 L of breast milk in the first 24 h and that the activity concentration in the treated mother's breast milk is a factor of 10 lower than the trace amounts detected in the urine, that is, 5 × 10−6 MBq/mL. Second, assume that all of this activity is ingested by the baby. As determined by the method reported by Stabin and Breitz (18), a nursing infant theoretically could ingest 0.001 MBq of the free 90Y. In this case, the effective dose to the nursing infant would be 0.02 mSv, on the basis of MIRDOSE3.1 with the newborn anthropomorphic mathematic phantom (19). The lower large intestine wall is the GI tract organ that would receive the highest β-dose, 0.1 mGy. Because an effective dose of 1 mSv to the infant is taken to be the cutoff dose criterion for recommending interruption or cessation of breast-feeding, the calculated radiation-absorbed dose is minimal and would not require any interruption of breast-feeding. Note that this dose estimate represents a worst-case scenario, because no 90Y activity has been detected in the blood and, therefore, the β-dose to the infant may in reality be much lower.
The second hazard is that there may be external radiation doses to the nursing infant from proximity to the mother before the radionuclides have cleared from her body. An interesting phantom study was performed to evaluate the external doses to the nursing infant (20). Activity was modeled in various maternal organs and tissues, and 3 model geometries were studied: infant on lap of mother, infant at breast of mother, and infant on shoulder of mother. The highest doses to the infant in these cases were determined to be from activity in the mother's whole body (uniformly distributed), liver, and thyroid, respectively.
Equation 2 can be used to estimate the dose that a nursing infant is likely to receive from external exposure to a breast-feeding mother released after receiving an administered activity of 90Y-microspheres of 3 GBq, assuming an occupancy factor of 0.167 at a conservative distance of 15 cm (center of liver to anterior surface of infant at level of umbilicus) for a nursing infant at the breast (corresponding to 4 h of nursing) and an occupancy factor of 0.083 at a distance of 30 cm (corresponding to 1 h on the mother's lap and 1 h on the mother's shoulder per day to total decay), as follows: The dose to the nursing infant obviously depends on its position with respect to the mother and the time spent in nursing and cuddling activities. We believe that our dose estimate is conservative because we have assumed conservative scenarios. A 1-mSv dose likely would not be reached with administered activities of 90Y-microspheres of less than approximately 17 GBq. Such administered activities are not likely to be encountered with resin or glass microspheres. For patients who receive a glass microsphere activity of 9 GBq, the estimated dose attributable to bremsstrahlung radiation would be higher by a factor of 3, or 0.53 mSv. Thus, the external dose, like the internal dose, would also be minimal, and it certainly would be extremely conservative to advise patients to avoid close contact with children or pregnant women or even to discontinue breast-feeding, if a breast-feeding patient were actually administered 90Y-glass microspheres with an activity as high as 9 GBq.
The dose to the nursing infant obviously depends on its position with respect to the mother and the time spent in nursing and cuddling activities. We believe that our dose estimate is conservative because we have assumed conservative scenarios. A 1-mSv dose likely would not be reached with administered activities of 90Y-microspheres of less than approximately 17 GBq. Such administered activities are not likely to be encountered with resin or glass microspheres. For patients who receive a glass microsphere activity of 9 GBq, the estimated dose attributable to bremsstrahlung radiation would be higher by a factor of 3, or 0.53 mSv. Thus, the external dose, like the internal dose, would also be minimal, and it certainly would be extremely conservative to advise patients to avoid close contact with children or pregnant women or even to discontinue breast-feeding, if a breast-feeding patient were actually administered 90Y-glass microspheres with an activity as high as 9 GBq.
Estimated Radiation Dose to Embryo or Fetus
Fetal radiation exposure is largely a theoretic situation, because radiomicrosphere treatment is contraindicated in pregnant women. However, should 90Y-microspheres be administered to a pregnant, or soon to be pregnant, woman, fetal radiation exposure is a possibility. The only NRC regulation relevant to this situation is the reporting requirement, pursuant to 10 CFR 35.3047, that requires licensees to report any dose to an embryo or fetus that exceeds a 50-mSv dose equivalent resulting from the administration of by-product material to a pregnant woman.
One study reported S values for bremsstrahlung doses to various target organs from a uniform source of 90Y activity in the liver (21). That study concluded that the bremsstrahlung dose from an organ to itself is very small compared with that from the β-dose, but the dose to other target organs may not always be negligible, especially if large amounts of 90Y are involved. For activity distributed in the liver, the bremsstrahlung dose to the liver is approximately 0.2% of the β-dose, and the 2 organs expected to receive the largest bremsstrahlung dose are the gallbladder and the adrenal glands. For an administered activity of 90Y-microspheres of 3 GBq, confined to the liver, these organs would receive radiation-absorbed doses of 57 and 28 mGy, respectively. A fetus (or embryo) would receive a radiation dose of 2 mGy, as determined with the liver-to-uterus S value as a surrogate (21). A higher administered activity of glass microspheres (9 GBq) could potentially deliver a fetal radiation dose of 6 mGy. These radiation doses are not associated with any known effects on the embryo or fetus (22).
Estimated Radiation Dose to Gonads
It may be advisable for patients receiving high radiation doses to the gonads (>250 mGy) to wait for several months after such exposures before conceiving offspring. It is not known whether the interval between irradiation of the gonads and conception has a marked effect on the frequency of genetic changes in human offspring, but at times a physician may need to advise a patient to defer conception for several months (22). The gonads, that is, the ovaries and testes, in patients undergoing therapy with 90Y-microspheres may receive estimated bremsstrahlung doses of 2.4 and 0.2 mGy, respectively, from an administered activity of 3 GBq, confined to the liver. These doses are 100- and 1,000-fold, respectively, lower than that recommended before advising any patient to defer conception.
There is no legitimate radiation protection reason to advise patients to abstain from sex after radiomicrosphere treatment. The recommended advice after 90Y-ibritumomab tiuxetan treatment is to avoid pregnancy by using condoms for 1 wk (23). The maximum treatment activity is 1,184 MBq; this activity is lower than the administered activities of 90Y-microspheres. However, 90Y-ibritumomab tiuxetan can more readily reach body fluids than 90Y-microspheres. Condom use is not necessary for glass microsphere–treated patients or beyond the first 24 h for resin microsphere–treated patients.
Instructions Appropriate for Released Patient
Standard universal precautions to avoid contact with body fluids are all that are required to ensure minimal doses to all others exposed to patients receiving 90Y-microsphere treatment. Relatively simple instructions have been recommended for outpatient 90Y-ibritumomab tiuxetan treatment (23): for 3 d, clean up any spilled urine and dispose of any body fluid–contaminated materials (e.g., flush down toilet or place in household trash), and wash hands after using toilet; and for a period of 1 wk, use condoms for sexual relations. Body fluid radioactivity is not problematic for 90Y-microspheres; therefore, patient release instructions involving hand washing or cleaning up of any contaminated materials are not necessary for glass microsphere–treated patients or for longer than 24 h for resin microsphere–treated patients. For the latter group, it may be prudent to instruct patients to wash their hands after voiding, to have men sit to urinate, and to dispose of any body fluid–contaminated materials (e.g., flush down toilet or place in household trash) during the first day.
General Radiation Safety Considerations
Routine radiation surveys, as with any other therapeutic radionuclide administration, must be performed at the end of each day in all areas in which the 90Y-microsphere treatment was prepared or administered (pursuant to 10 CFR 35.70). Records of each survey must be retained for 3 y (pursuant to 10 CFR 35.2070). Procedures need to be in place to deal with a radioactive spill or contamination incident. Appropriate procedures must also be in place to ensure the proper storage and waste disposal of any radioactive items (pursuant to 10 CFR 35.92), keeping in mind the potential for longer decay-in-storage times because of the presence of the previously mentioned long-lived radiocontaminants potentially present, and records must be kept (pursuant to 10 CFR 35.2092).
CONCLUSION
Because 90Y is a pure β-emitter, no burdensome radiation safety precautions are necessary for health care workers, patients, or their families. Shielding is accomplished with plastic and acrylic materials during dose preparation and administration; lead should be avoided because of the external exposure risk attributable to bremsstrahlung production. Radiomicrosphere treatment is an outpatient therapy because the potential radiation doses to individuals likely to be exposed to a 90Y-microsphere–treated patient are insignificant in most cases. The estimated bremsstrahlung doses to a patient's gonads and to an embryo or fetus were determined to assess the potential for any possible radiation-related consequences. These latter estimated doses were negligible. No release instructions are required for glass microsphere–treated patients (unless activities higher than 9 GBq are administered), whereas patients receiving resin microspheres may be given simple precautionary instructions for the first 24 h only, involving careful bathroom hygiene practices and condom use.
The bremsstrahlung component of the decay scheme of the pure β-emitter 90Y has traditionally been ignored in internal and external dose calculations, generally because of the belief that the contribution of this component is negligible. Because the production of in vivo bremsstrahlung with the high-energy pure β-emitting radionuclides used for therapeutic purposes is sufficient to permit external detection and imaging (24–26), the resulting potential external radiation hazard needed to be evaluated. This hazard has been systematically discussed in this article. The dose calculations performed with regard to patient release indicated that, consistent with regulatory requirements, patients receiving 90Y-microsphere therapy can be immediately released without the need for radiation safety instructions on actions aimed at maintaining doses to others as low as reasonably achievable, as these doses are all minimal and well below regulatory limits.
Footnotes
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication July 18, 2007.
- Accepted for publication September 17, 2007.







