Abstract
We evaluated the diagnostic accuracy of PET with l-methyl-11C-methionine (11C-MET) for the differentiation of recurrent brain tumors from radiation necrosis. Methods: Seventy-seven patients who had been previously treated with radiotherapy after primary treatment for metastatic brain tumor (n = 51) or glioma (n = 26) were studied to clarify the diagnostic performance of 11C-MET PET in differentiating between recurrent brain tumors and radiation necrosis. A total of 88 PET scans with 11C-MET were obtained; sometimes more than one scan was obtained when there was an indication of recurrent brain tumor or radiation necrosis. A definitive diagnosis was made on the basis of pathologic examination for recurrent brain tumors and on the basis of pathologic examination or clinical course for radiation necrosis. Several indices characterizing the lesions were determined; these included mean and maximum standardized uptake values (SUVmean and SUVmax, respectively) and the ratios of lesion uptake to contralateral normal frontal-lobe gray matter uptake corresponding to the SUVmean and the SUVmax (L/Nmean and L/Nmax, respectively). Receiver-operating-characteristic (ROC) curve analysis was used to determine the optimal index of 11C-MET PET and cutoff values for the differential diagnosis of tumor recurrence and radiation necrosis. Results: The values of each index of 11C-MET PET tended to be higher for tumor recurrence than for radiation necrosis. There were significant differences between tumor recurrence and radiation necrosis in all of the indices except for the L/Nmax for glioma. ROC analysis indicated that the L/Nmean was the most informative index for differentiating between tumor recurrence and radiation necrosis. An L/Nmean of greater than 1.41 provided the best sensitivity and specificity for metastatic brain tumor (79% and 75%, respectively), and an L/Nmean of greater than 1.58 provided the best sensitivity and specificity for glioma (75% and 75%, respectively). Conclusion: 11C-MET PET can provide quantitative values to aid in the differentiation of tumor recurrence from radiation necrosis, although these values do not appear to be absolute indicators. Quantitative analysis of 11C-MET PET data may be helpful in managing irradiated brain tumors.
Primary treatment of brain tumors usually consists of a combination of surgery, radiotherapy, and chemotherapy. Postradiation reactions in the central nervous system can occur after conventional radiotherapy and stereotactic radiosurgery (SRS) (1). Radiation necrosis after the aggressive use of irradiation for malignant brain tumors appears to be more common than previously estimated (2). Differentiating between recurrent brain tumors and radiation necrosis, however, is often difficult with conventional diagnostic imaging techniques, such as MRI (3). This is an unsolved issue in managing irradiated brain tumors.
Recently, several imaging modalities, such as MR spectroscopy (4–6), SPECT with 201Tl-chloride (201Tl) (7), and PET with various radiotracers (8), were used to differentiate tumor recurrence from radiation necrosis in patients with irradiated brain tumors. However, a noninvasive method for differential diagnosis still remains a challenge.
The present study was conducted to evaluate the diagnostic accuracy of PET with l-methyl-11C-methionine (11C-MET) for the differentiation of recurrent metastatic brain tumors or gliomas from radiation necrosis in a relatively large population of patients and to determine the optimal index and cutoff values for differentiating between recurrent tumors and radiation necrosis.
MATERIALS AND METHODS
Patients
From March 1995 to December 2006, 216 patients with irradiated brain tumors (metastatic brain tumor or glioma) and with a clinical indication (clinical symptoms and MRI findings) of recurrent brain tumors or radiation necrosis underwent 11C-MET PET. Among this group, patients whose follow-up was insufficient for a definitive diagnosis or who underwent additional radiotherapy without a pathologic confirmation were excluded. Finally, 77 patients (46 males and 31 females; 14–83 y old [range], 54.1 ± 14.5 y old [mean ± SD]) whose definitive diagnosis was confirmed were included in this study. Some of these patients were included in previous studies (9,10). The primary lesions were 51 metastatic brain tumors (lung, n = 36; colon, n = 6; kidney, n = 3; breast, n = 2; ovary, n = 1; testis, n = 1; unknown, n = 2) and 26 gliomas (World Health Organization histopathologic classification: grade II, n = 6; grade III, n = 6; grade IV, n = 14). Overall, 88 PET scans were obtained (56 for metastatic brain tumors and 32 for gliomas). Before the PET studies, all patients had undergone irradiation (either conventional radiotherapy or SRS) after primary treatment for gliomas or metastatic brain tumors. All but 4 patients with metastatic brain tumors underwent SRS without conventional radiotherapy. The remaining 4 patients underwent conventional radiotherapy in addition to SRS. All patients with gliomas underwent conventional radiotherapy with or without SRS. The mean intervals between irradiation and PET scans were 17.2 mo for metastatic brain tumors and 36.1 mo for gliomas.
A definitive diagnosis of recurrent tumor or radiation necrosis was determined as follows. Recurrence was defined as a case in which pathologic diagnosis was confirmed by tumor resection or biopsy. We excluded patients in whom recurrent tumor had been strongly indicated on the basis of clinical presentation or the results of 11C-MET PET and in whom additional radiotherapy without pathologic diagnosis had been performed. Diagnosis of radiation necrosis was based on pathologic examination or clinical course. Cases in which lesions showed spontaneous shrinkage or remained stable in size on MRI after a long-term follow-up (more than 6 mo) were assumed to be radiation necrosis.
PET
PET was performed with a HEADTOME-IV PET scanner (Shimadzu) with a spatial resolution of 4.5 mm (full width at half maximum) and a slice thickness of 6.5 mm (14 slices) (11). Patients were placed in the scanner so that slices parallel to the orbitomeatal line could be obtained. During a period of fasting, patients were injected intravenously with 11C-MET at 6 MBq/kg over 30 s. After a transmission scan was obtained, a static scan of 10 min was begun 20 min after the injection.
Imaging Analysis
The scans were interpreted by 2 experienced nuclear medicine radiologists. The PET images were reconstructed by use of measured attenuation correction. Any area with a level of uptake higher than that in the adjacent normal tissue was selected as the region of interest (ROI) for lesions; any cystic or necrotic portions were avoided. As a normal control, several circular ROIs with a diameter of 10 mm were located over the gray matter of the contralateral frontal lobe. If no abnormality could be detected with PET, then a circular ROI of the same size was located over the area corresponding to the abnormality on MRI.
Activity counts were normalized to injected dose per kilogram of patient body weight (standardized uptake value [SUV]), as follows: SUV = [(pixel count/pixel volume)/(injected radioisotope activity/body weight)] × calibration factor. The mean pixel count of the SUV (SUVmean) and the maximum pixel count of the SUV (SUVmax) were generated over the ROI. The lesion-to-normal tissue (L/N) ratios were generated by dividing the SUVmean of the lesion by the SUVmean of the contralateral normal frontal-lobe gray matter (L/Nmean) and by dividing the SUVmax of the lesion by the SUVmean of the contralateral normal frontal-lobe gray matter (L/Nmax).
Statistical Analysis
The values of each index (SUVmean, SUVmax, L/Nmean, and L/Nmax) for tumor recurrence and radiation necrosis for metastatic brain tumors and gliomas were compared separately by use of the Mann–Whitney nonparametric test. Receiver-operating-characteristic (ROC) curve analysis was used to determine the optimal index of 11C-MET PET and cutoff values for the differential diagnosis of tumor recurrence and radiation necrosis. Significance was defined as a probability value of less than 0.05.
RESULTS
Table 1 summarizes the definitive diagnosis of the lesions. All of the tumor recurrence cases and 6 of the radiation necrosis cases were confirmed by pathologic examination. Illustrative images from patients with tumor recurrence and radiation necrosis are shown in Figures 1 and 2.
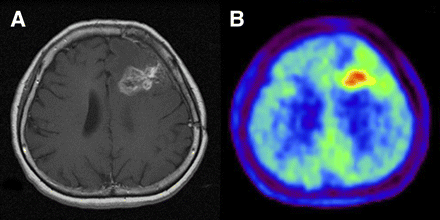
Imaging of 49-y-old woman who had been previously treated for glioblastoma multiforme with tumor resection and conventional radiotherapy at dose of 60 Gy. (A) T1-weighted MR image with contrast medium, obtained 13 mo after initial surgery, showing contrast-enhanced lesion in left frontal lobe. (B) 11C-MET PET image showing obvious accumulation of tracer corresponding to abnormality on MR image. L/Nmean was 1.70. Recurrent tumor was pathologically confirmed by second surgery.
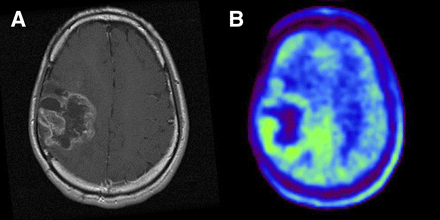
Imaging of 25-y-old man who had been previously treated for anaplastic astrocytoma with tumor resection and conventional radiotherapy at dose of 60 Gy. (A) T1-weighted MR image with contrast medium, obtained 21 mo after initial surgery, showing ringlike enhancement of lesion in right frontoparietal area. (B) 11C-MET PET image showing slight accumulation of tracer corresponding to abnormality on MR image. L/Nmean was 1.44. Gliosis without tumor was pathologically demonstrated by second surgery.
Definitive Diagnosis of Lesions
Comparison of Each Index for Tumor Recurrence and Radiation Necrosis
The PET scan data are shown in Table 2. The values of each index tended to be higher for tumor recurrence than for radiation necrosis. With respect to metastatic brain tumors, there were significant differences in all of the indices between tumor recurrence and radiation necrosis (P = 0.000048 for SUVmean, P = 0.0023 for SUVmax, P = 0.00011 for L/Nmean, and P = 0.0019 for L/Nmax) (Fig. 3A). With respect to gliomas, there were significant differences in all of the indices except for the L/Nmax between tumor recurrence and radiation necrosis (P = 0.017 for SUVmean, P = 0.036 for SUVmax, and P = 0.0079 for L/Nmean). There was no significant difference in the L/Nmax (P = 0.052) (Fig. 3B).
(A) Box-and-whisker plots of each 11C-MET PET index for metastatic brain tumors. Horizontal bars inside boxes indicate median values. Error bars indicate farthest points that are not outliers. There were significant differences in all indices between tumor recurrence and radiation necrosis (P = 0.000048 for SUVmean, P = 0.0023 for SUVmax, P = 0.00011 for L/Nmean, and P = 0.0019 for L/Nmax). (B) Box-and-whisker plots of each 11C-MET PET index for gliomas. Horizontal bars inside boxes indicate median values. Error bars indicate farthest points that are not outliers. There were significant differences in all indices except for L/Nmax between tumor recurrence and radiation necrosis (P = 0.017 for SUVmean, P = 0.036 for SUVmax, P = 0.0079 for L/Nmean, and P = 0.052 for L/Nmax).
Quantitative Analysis of 11C-MET PET Data
ROC Analysis of Each Index of 11C-MET PET
Figure 4 shows the ROC curves for the L/Nmean, L/Nmax, SUVmean, and SUVmax for each type of brain tumor. The areas under the ROC curve (Az) for metastatic brain tumors were 0.780 for the L/Nmean, 0.747 for the L/Nmax, 0.760 for the SUVmean, and 0.696 for the SUVmax (Fig. 4A). The Az for gliomas were 0.745 for the L/Nmean, 0.701 for the L/Nmax, 0.725 for the SUVmean, and 0.652 for the SUVmax (Fig. 4B). These data indicate that the L/Nmean is the most informative index for differentiating between tumor recurrence and radiation necrosis.
(A) ROC curve of each index for metastatic brain tumors. Az were 0.780 for L/Nmean, 0.747 for L/Nmax, 0.760 for SUVmean, and 0.696 for SUVmax. (B) ROC curve of each index for gliomas. Az were 0.745 for L/Nmean, 0.701 for L/Nmax, 0.725 for SUVmean, and 0.652 for SUVmax.
An L/Nmean of 1.41 provided the best sensitivity and specificity for metastatic brain tumors, 79% and 75%, respectively, and an L/Nmean of 1.58 provided the best sensitivity and specificity for gliomas, 75% and 75%, respectively (Table 3).
Optimal Cutoff Values and Diagnostic Accuracy
DISCUSSION
In the field of PET, 18F-FDG has received the most study. Patronas et al. reported that among 5 cases of glioma, 2 cases of radiation necrosis and 3 cases of recurrent tumor were correctly distinguished by PET with 18F-FDG (12). Since then, 18F-FDG PET has been widely applied for the evaluation of irradiated brain tumors. Most of the earlier studies supported the use of 18F-FDG PET for differentiating between recurrent tumors and radiation necrosis with a high accuracy (2,13–17). On the other hand, more recent studies indicated some limitations of 18F-FDG PET for that purpose (18–23). A comparative study in 19 patients with brain tumors that were evaluated with both 201Tl SPECT and 18F-FDG PET found the sensitivity and the specificity to be 81% and 40% for 18F-FDG PET and 69% and 40% for 201Tl SPECT (19). That study failed to demonstrate a significant difference between the 2 modalities in terms of sensitivity or specificity (19). Wong et al. noted the limitation of 18F-FDG PET for the differential diagnosis of recurrent high-grade tumors and radiation necrosis (23). Chao et al. used 18F-FDG PET with MRI coregistration to evaluate 47 patients with various types of brain tumors treated with SRS (20). They demonstrated that 18F-FDG PET without MRI coregistration provided a sensitivity of 65% and a specificity of 80% but that the addition of MRI coregistration improved the sensitivity of 18F-FDG PET to up to 86% in patients with metastatic brain tumors (20).
PET with amino acid tracers can be an alternative. 11C-MET is considered to accumulate preferentially in tumor tissue, with a low level of accumulation in normal brain tissue, providing good contrast to highlight tumor uptake; in contrast, 18F-FDG accumulates preferentially in normal gray matter. Several previous reports showed that PET with 11C-MET is effective for differentiating between recurrent gliomas and radiation-induced changes and can provide early detection of a recurrence (9,24,25). Tsuyuguchi et al. previously suggested that 11C-MET PET is also useful for differentiating between metastatic brain tumor recurrence and radiation necrosis after SRS (10). In contrast, some authors have discussed the limitations of 11C-MET PET in differentiating between recurrent tumors and radiation necrosis (13,26). To our knowledge, this is the first clinical study to evaluate the diagnostic accuracy of 11C-MET PET in a large number of patients and to compare several indices based on 11C-MET PET in terms of their abilities to aid in the differential diagnosis of recurrent brain tumors and radiation necrosis.
More recently, Chen et al. studied patients with various brain tumors by using the amino acid tracer 3,4-dihydroxy-6-18F-fluoro-l-phenylalanine (18F-FDOPA) (27). They demonstrated the superiority of 18F-FDOPA PET over 18F-FDG PET for imaging low-grade tumors and evaluating recurrent brain tumors (27). The sensitivity and the specificity of 18F-FDOPA PET in their study were 98% and 86%, respectively (27); these results are more favorable than our results obtained with 11C-MET.
When comparing diagnostic accuracies, however, one must consider that definitions of outcome and patient population are not uniform among published studies, even though these factors can affect the results. Many cases in which tumor recurrence was strongly suggested by the clinical course and high level of uptake of 11C-MET were excluded from the present study because the patients had received additional radiotherapy without pathologic confirmation. Such cases seem to have been included as tumor recurrence in some previous studies (4,20,22,27). Among the patients excluded from the present study, we found that 24 patients with metastatic brain tumors (25 11C-MET PET scans) had undergone additional SRS on the basis of the clinical course. Provided that those patients represented cases of true recurrence, the sensitivity and the specificity in patients with metastatic brain tumors would be 82% and 75%, respectively. This technique appeared to be more sensitive but less specific than 18F-FDG PET alone, without MRI coregistration, that is, a sensitivity of 65% and a specificity of 80% (20). Moreover, all of the patients in the present study had received irradiation and were thought to have recurrence or radiation necrosis, whereas several previous studies included some newly diagnosed patients. Hence, we believe that the present study does not necessarily indicate that 11C-MET PET is less informative.
However, our results suggest the limitation of 11C-MET PET for evaluating irradiated tumors. Quantitative analysis in the present study demonstrated that some necrotic tissues also had a high level of 11C-MET accumulation. This can be a factor that reduces the specificity of 11C-MET PET. Doyle et al. studied 19 patients with irradiated brain tumors by using PET with 82Rb, a sensitive marker for blood–brain barrier (BBB) damage (17). They reported that some necrotic tissues showed a high level of 82Rb accumulation, indicating a disrupted BBB (17). Because 11C-MET is thought to accumulate in tissue with a disrupted BBB (13,28), the level of 11C-MET uptake in necrotic tissue should be elevated. Such a disadvantage of 11C-MET was previously described by Roelcke et al. (28). They compared the levels of uptake of 11C-MET and 82Rb in 30 patients with various brain tumors and demonstrated that passive diffusion through the BBB may play a role in 11C-MET accumulation. They concluded that the value of 11C-MET PET is limited when disruption of the BBB is present.
L/N ratios were used for differentiating between tumors, mostly low-grade gliomas, and nontumoral lesions in a published study with 11C-MET PET (29). The authors of that study found that the optimal cutoff value was 1.47, providing a sensitivity of 76% and a specificity of 87% (29). A noteworthy and interesting finding of the present study was that ROC analysis showed distinct optimal cutoff values for metastatic brain tumors (L/Nmean: 1.41) and gliomas (L/Nmean: 1.58). We believe that this finding was attributable to the difference in the mode of irradiation as well as the histology. Most of the patients with metastatic brain tumors in the present study had undergone SRS without conventional radiotherapy, whereas all of the patients with gliomas had undergone conventional radiotherapy with or without SRS. We believe that 11C-MET metabolism can be decreased by irradiation even in normal gray matter, so that L/N ratios can increase as the SUV of the normal gray matter (denominator) decreases. In fact, we found that the SUVmean of the normal frontal-lobe gray matter in patients with gliomas tended to be lower than that in patients with metastatic brain tumors, although the difference was not statistically significant (P = 0.11). This observation was contrary to a previous report that a suppression effect was not observed in the contralateral cortex in 3 patients with brain tumors after radiotherapy (30). Another possible reason for the difference in cutoff values is the difference between metastatic brain tumors and gliomas in terms of the pathophysiology of necrotic tissue. Previous studies with pathologic examination suggested that the necrotic tissue seen with metastatic brain tumors consisted of inflammation without reactive gliosis (10), whereas the necrotic tissue seen with gliomas consisted of gliosis with neither inflammation nor vascular proliferation (9). This difference in pathology can affect the accumulation of 11C-MET as well.
CONCLUSION
We have demonstrated that certain quantitative values determined from 11C-MET PET can differentiate tumor recurrence from radiation necrosis. The L/Nmean of 11C-MET PET may be the most valuable index for this differential diagnosis for both metastatic brain tumors and gliomas. We suggest that quantitative analysis of 11C-MET PET data is helpful in managing irradiated brain tumors, although the quantitative values did not appear to be absolute indicators. When interpreting 11C-MET PET data, one should consider that cutoff values may differ among tumors and that irradiation may affect 11C-MET metabolism.
Acknowledgments
The authors thank Dr. Susumu Shiomi and Dr. Ichiro Sunada for their valuable assistance during this study and technologists Yoshihiro Shimonishi and Hiroyuki Tsushima at the Central Radiology Department of Osaka City University Medical School for their technical support in PET acquisition.
Footnotes
-
COPYRIGHT © 2008 by the Society of Nuclear Medicine, Inc.
References
- Received for publication October 10, 2007.
- Accepted for publication January 22, 2008.