Abstract
The purpose of this study was to explore the uptake of the synthetic amino acid analog PET radiotracer anti-3-18F-FACBC (18F-fluciclovine) in breast lesions with correlation to histologic and immunohistochemical characteristics. Methods: Twelve women with breast lesions underwent 45-min dynamic PET/CT of the thorax after intravenous administration of 366.3 ± 14.8 (337.44–394.05) MBq of 18F-fluciclovine. Uptake in the primary lesions at 4 representative time points (5, 17, 29, and 41 min) after injection were correlated with histologic, imaging, and clinical findings. The significance of differences in SUVmax and tumor-to-background ratios between malignant and benign tissue were calculated. Correlations of activity to histologic and immunohistochemical cancer subtypes were made including Ki-67 intensity and Nottingham grade (NG). Results: There were 17 breast lesions (4 benign, 13 malignant) including 7 of 13 invasive ductal, 5 of 13 invasive lobular, and 1 of 13 metaplastic carcinomas. There was a significant difference in mean SUVmax ± SD of malignant (6.2 ± 3.2, 6.0 ± 3.2, 5.7 ± 2.8, and 5.6 ± 3.0) versus benign (1.3 ± 0.6, 1.2 ± 0.5, 1.2 ± 0.6, and 1.1 ± 0.5) lesions at 5, 17, 29, and 41 min, respectively (all P ≤ 0.0001). Tumor-to-background (aorta, normal breast, and marrow) ratios were also significantly higher in malignant than benign breast lesions (all P ≤ 0.02). The highest 18F-fluciclovine activity seems to be present in triple-negative and NG3 subtypes. Across time points, quantitative Ki-67 had weak positive correlation with SUVmax (R1 = 0.48 [P = 0.03], R2 = 0.44 [P = 0.03], R3 = 0.46 [P = 0.03], R4 = 0.43 [0.06]). In 7 patients, 18F-fluciclovine PET visualized locoregional and distant spread including that of lobular cancer, though identification of hepatic metastases was limited by physiologic background activity. Conclusion: The uptake characteristics of 18F-fluciclovine are reflective of the histologic and immunohistochemical characteristics in suspected breast lesions with greater activity in malignant versus benign etiology. The data from this exploratory study may be useful to design future studies using 18F-fluciclovine PET for breast tumor imaging as well as for detection of locoregional and distant spread.
Breast cancer is the most common cancer and the second leading cause of cancer-related deaths in women (1). Histologic and molecular characteristics of breast cancer have significant effects on therapeutic decisions as well as disease-free and overall survival (2). Mammography, ultrasound, and MRI form the backbone of breast imaging (3–6).
18F-FDG PET has also assumed an important role in whole-body staging, recurrent disease detection, and therapy response monitoring (7). Molecular breast imaging with 18F-FDG positron emission mammography and 99mTc-sestamibi are also being explored (3,8,9). These have limitations including differentiation of malignancy from posttherapy effects and challenges with certain histologic subtypes such as lobular cancer, which accounts for 10% of invasive breast cancers. Thus, molecular imaging targeting receptors or other aspects of the metabolome are under investigation (8,9). Because amino acid metabolism is upregulated in breast cancer, molecular imaging using natural or synthetic amino acid radiotracers is a potentially attractive approach (9,10).
Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (18F-FACBC or 18F-fluciclovine) is a synthetic amino acid analog transported via system L (L-type amino acid transporter 1 [LAT1]) and alanine/serine/cysteine transporter 2 (ASCT2) amino acid transporters and has shown promise in the imaging of prostate and other cancers (11). Liang reported 18F-fluciclovine uptake in breast carcinoma cell lines, which correlated with malignant potential, as well as in orthotopic MDA-MB-231 breast carcinoma xenografts (12). In this study, we aimed to explore uptake of 18F-fluciclovine in suspected breast lesions, report associations between uptake and histologic as well as immunohistochemical characteristics of breast carcinoma, and describe activity within locoregional or remote tumor spread.
MATERIALS AND METHODS
Patient Selection
The institutional review board at Emory University approved this study, which is Health Insurance Portability and Accountability Act–compliant, and all subjects signed a written informed consent form. Twelve female patients 18 y or older with breast lesions naïve to therapy and about to undergo biopsy or who were at least 1 wk postbiopsy were recruited and underwent 18F-fluciclovine PET/CT between September 2012 and January 2015 (NCT01659645).
18F-Fluciclovine PET/CT Imaging
Preparation of 18F-fluciclovine under Investigational New Drug Application 72,437 was completed via the FastLab Cassette System (GE Healthcare) or via automated synthesis (13). All subjects fasted for at least 4 h to stabilize plasma amino acid levels (14). A CT of the thorax (80–120 mA; 120 kVp) was performed then, 366.3 ± 14.8 (337.44–394.05) MBq of 18F-fluciclovine were injected intravenously, and a 45-min dynamic list-mode PET acquisition at 1 table position encompassing the primary breast lesions was completed in a Discovery 690 PET/CT scanner (GE Healthcare). Data were reconstructed into 4-min time frames and transferred to a MIMVista workstation (MIM Software) for analysis. Four patients also underwent clinical 18F-FDG PET performed on the same scanner after intravenous injection of 439.84 ± 37 MBq of 18F-FDG and an uptake time of approximately 60 min. Blood glucose ranged from 89 to 104 mg/dL in these patients. 18F-Fluciclovine and 18F-FDG scans were obtained at an average interval ± SD of 12 ± 8 d (range, 1–21 d).
Safety Evaluation
Vitals signs were monitored during and immediately after the 18F-fluciclovine scan. Complete metabolic profile, complete blood count, and urinalysis just before and approximately 1 wk after the 18F-fluciclovine PET scan were obtained, and no attributable adverse events were noted. Dosimetry and phase 1 safety data have already been reported (15–17).
Image Analysis
A board-certified nuclear radiologist unmasked to all correlative imaging and clinical information using the 3-dimensional PET-Edge tool and a custom workflow (MIM Software) drew target and background regions of interest. After the regions of interest were drawn, the values of the maximum voxel (SUVmax), average voxel (SUVmean), and average of all voxels within a fixed spheric ROI of 1 cm3 centered on the most metabolically active part of the lesion (SUVpeak) were recorded at representative time points of 5–8 min, 17–20 min, 29–32 min, and 41–44 min (referenced as 5, 17, 29, and 41 min in this article). The SUVmean of background structures (blood pool at aortic arch, normal breast tissue and marrow at T5 vertebrae) were also measured using at least a 1-mL spheric region of interest best conforming to the background structure. For normal breast tissue, regions either contralateral or most distant from suspect lesions were chosen. Images were scrutinized for marker clips and, if present, were correlated to surgical reports for associations between imaging and histologic sampling. In ill-defined or multifocal breast lesions or in a nodal conglomerate, the most representative region was measured.
Histologic Analysis
Histologic examination of biopsy specimens was performed by a board-certified breast pathologist to evaluate the Nottingham grade (NG1, NG2, and NG3). Immunohistochemical analysis was performed for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67, using Dako's HERCEPTEST and MIB-1 kits, respectively. ER and PR expression was positive if 1% or more of the tumor nuclei were stained (18). HER2 was negative if staining was 0 or 1+, equivocal if 2+, and positive if 3+ per American Society of Clinical Oncology/College of American Pathologists guidelines. We analyzed HER2 equivocal cases by fluorescence in situ hybridization study to determine HER2 gene amplification. Ki-67 staining was scored as the number of positively stained nuclei as a percentage of the total tumor cells and reported in 3 categories: low, ≤10%; intermediate, >10% to <14%; and high, ≥14%.
Statistical Methods
A 2-tailed t test was used to compare the means of SUVmax in malignant with that of benign breast tumors and SUVmax of benign breast tumors with that of normal breast tissue across time points. ANOVA was used to compare the means of SUVmax of malignant lesions based on Nottingham grades, molecular subtypes, and Ki-67. A Pearson correlation coefficient was used to determine the correlation between SUVmax and Ki-67 intensity (%) in malignant breast tumors. Correlation coefficient (R) was interpreted as very strong, 0.9–1.0; strong, 0.7 to −<0.9; moderate, 0.5 to −<0.7; weak, 0.3 to −< 0.5; and none, < 0.3. All P values were 2-sided and statistically significant if less than 0.05. Statistical analysis was done using IBM SPSS Statistics 22 and Microsoft Excel 2010.
RESULTS
Demographics
Twelve women (11 postmenopausal and 1 premenopausal) with an average age ± SD of 64 ± 12 y (range, 49–89 y) were recruited. At diagnosis, 9 of 12 patients had primary breast cancer, and 3 of 12 had local disease recurrence. In these 12 women, 17 breast lesions (13 malignant and 4 benign) were analyzed. Histologic verification was via ultrasound-guided core biopsy except for 1 benign lesion confirmed on conventional imaging. Six of 12 biopsies were done within 1 d after 18F-fluciclovine PET/CT whereas 6 of 12 were done from 8–35 d before 18F-fluciclovine PET/CT. Demographic details and results of histologic and immunohistochemical analysis are provided in Table 1.
18F-Fluciclovine Activity Correlated to Demographic, Histologic, and Immunohistochemical Characteristics
18F-Fluciclovine Kinetics
On the basis of full dynamic analysis, uptake of 18F-fluciclovine peaked approximately 4 min after administration in 10 of 13 malignant lesions followed by a gradual clearance of 5%/min on average across these subjects. In the remaining 3 of 13 malignant lesions, the time to peak was the same, but the washout was substantially slower at 0.5%/min. There did not seem to be a relationship between these patterns and tumor subtype. Ratios of malignant lesions to the background structures of marrow and blood reached a plateau at between 6 and 10 min after administration. Malignant–to–normal breast tissue ratios decreased over time at 0.8%/min primarily because of a slower washout of normal tissue than of the malignant lesions.
18F-Fluciclovine Activity and Histologic Characteristics of Breast Lesions
Malignant breast lesions had significantly higher uptake than benign lesions, with higher tumor-to-background ratios (Fig. 1; Table 2). SUVmax in benign breast lesions was not significantly different from that of normal breast tissue (Table 2). Figure 2 is an example of uptake in benign and malignant breast lesions.
18F-Fluciclovine uptake in breast tumors (A), tumor-to-aorta ratios (P < 0.002) (B), tumor–to–normal breast ratios (P < 0.0004) (C), and tumor-to-marrow ratios (P < 0.0001) (D) in malignant compared with benign breast lesions.
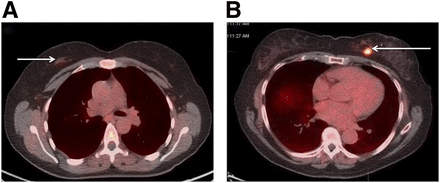
18F-Fluciclovine Uptake in Breast Lesions and Normal Breast Tissue
18F-Fluciclovine PET/CT images in benign ductal hyperplasia (arrow), SUVmax at 5 min = 2.0 (A), and in IDC (arrow), SUVmax at 5 min = 9.7 (B).
Of the malignant lesions, there were 7 of 13 invasive ductal carcinoma (IDC), 5 of 13 invasive lobular carcinoma (ILC), and 1 of 13 invasive metaplastic carcinoma with sarcomatous differentiation (IMC). The mean SUVmax (±SD) for IDC was 7.7 ± 2.8, 7.5 ± 2.8, 7.0 ± 2.4, and 6.8 ± 2.8, and for ILC it was 3.8 ± 1.8, 3.9 ± 1.7, 3.8 ± 1.5, and 3.9 ± 1.5 at 5, 17, 29, and 41 min, respectively. Although 18F-fluciclovine uptake in IDC and ILC were both higher than benign breast lesions (P ≤ 0.01 across time points), IDC had significantly higher uptake than ILC (P ≤ 0.02 across time points). Figure 3 is a scatterplot of IDC, ILC, and benign lesions. The IMC had an SUVmax of 8.6, 8.9, 8.9, and 9.1 across time points.
18F-Fluciclovine uptake in IDC, ILC, and benign breast lesions at 5 (A), 17 (B), 29 (C), and 41 (D) min.
There were 3 of 12 NG1, 5 of 12 NG2, and 4 of 12 NG3 tumors. NG is not applicable to IMC. Except at the first time point, NG3 had significantly higher uptake than NG2 (P = 0.06, 0.03, 0.03, and 0.03) and NG1 (P = 0.13, 0.04, 0.03, and 0.03) (Table 3). There was no significant difference in uptake between NG1 and NG2.
18F-Fluciclovine Uptake (SUVmax) Across Nottingham Grades and Receptor Status
18F-Fluciclovine Activity and Immunohistochemical Characteristics of Malignant Breast Tumors
Tumors were classified into standard subtypes based on immunohistochemical analysis: triple-negative (n = 2), HER2-positive type (HER2+; n = 1), and ER-positive/HER2-negative type (ER+/HER 2− [PR-positive [PR+] or PR-negative [PR−]; n = 10) (19). Triple-negative breast tumors demonstrated the highest mean uptake, followed by ER+/HER2− and lowest uptake in HER2+ type though without overall significance likely due to small sample size (Table 3). Figure 4 shows differential activity in a patient with triple-negative and ER+/HER2− tumors. Ki-67 intensity demonstrated positive weak linear correlation (RTime point) with SUVmax with R1 = 0.48 (P = 0.03), R2 = 0.44 (P = 0.03), R3 = 0.46 (P = 0.03), and R4 = 0.43 (P = 0.06).
18F-Fluciclovine PET/CT image in bilateral breast cancer showing higher uptake (SUVmax at 5 min = 11.7) in triple-negative IDC on right (arrow) compared with uptake (SUVmax at 5 min = 8.8) in ER+/HER2− IDC on left (arrow).
18F-Fluciclovine PET/CT and Locoregional and Distant Metastases
Histologic sampling occurred in 9 locoregional lymph nodes (4 benign and 5 malignant). Of the malignant nodes, 3 were ILC and 2 were IDC. At 5, 17, 29, and 41 min, respectively, ILC nodes had mean a SUVmax (±SD) of 6.2 ± 0.8, 5.4 ± 0.7, 4.8 ± 0.9, and 4.3 ± 0.7; IDC nodes had a mean SUVmax of 3.3 ± 2.6, 3.4 ± 2.2, 3.7 ± 2.6, and 4.0 ± 2.5; and benign nodes had mean a SUVmax of 1.5 ± 0.4, 1.0 ± 0.1, 0.9 ± 0.3, and 0.8 ± 0.3. Differences were not statistically significant, but sample size was small. Figure 5 shows 18F-fluciclovine uptake in a biopsy-proven ILC metastasis to axillary node reported negative on outside MRI and ultrasound. No internal mammary or more distant nodes were identified on 18F-fluciclovine or other imaging. In 1 patient, micrometastatic (≤2 mm) nodal disease was not detected on 18F-fluciclovine imaging, and in another patient a benign sentinel node could not be correlated to imaging.
CT (A) and 18F-fluciclovine PET/CT (B) images in left ILC. SUVmax at 5 min = 3.5 in primary mass (short arrow) and 5.6 in biopsy-proven metastasis to a 6 × 11 mm left axillary lymph node (long arrow).
In 1 patient with stage IV breast cancer, left axillary and skeletal involvement were well visualized on 18F-fluciclovine imaging but not enlarging hepatic metastases on CT. Mean 18F-fluciclovine SUVmax in 4 representative skeletal lesions was 11.1 ± 1.7, 10.5 ± 2.3, 9.6 ± 2.3, and 9.1 ± 1.7 across time points. Although SUVmax in a representative liver lesion was 7.6, 9.2, 10.3, and 10.5 across 4 time points, these lesions appeared relatively photopenic due to more intense physiologic hepatic background.
18F-Fluciclovine PET/CT Correlated with 18F-FDG PET/CT
Four patients also underwent clinical 18F-FDG PET/CT. 18F-Fluciclovine and 18F-FDG activity were compared in 8 biopsied lesions (5 malignant and 2 benign primary, 1 malignant node). The malignant lesions included 3 IDC, 2 ILC, and 1 IMC. For IDC, mean SUVmax at 5 min with 18F-fluciclovine was 8.7 ± 3.1 compared with 8.7 ± 4.2 for 18F-FDG. For ILC, mean SUVmax at 5 min with 18F-fluciclovine was 4.6 ± 3.5 compared with 2.6 ± 1.0 for 18F-FDG. Figure 6 is an example of uptake in an ILC intramammary nodal metastasis on 18F-fluciclovine and 18F-FDG PET/CT imaging. In the IMC, 18F-FDG had a higher uptake (SUVmax, 19.5) than 18F-fluciclovine (SUVmax 8.6 at 5 min) and both had similarly low activity in the necrotic center. For the benign breast lesions (fibrocystic change with focal ductal hyperplasia and posttherapy scar), SUVmax on 18F-fluciclovine imaging was 1.7 ± 0.7 at 5 min versus 2.1 ± 1.1 on 18F-FDG. Differences between 18F-fluciclovine and 18F-FDG activity were not significant, but the sample size was small.
Higher uptake on 18F-fluciclovine (SUVmax at 5 min = 7.1) (A) than 18F-FDG (SUVmax = 3.3) (B) PET/CT imaging in 2.1 × 1.1 cm right ILC metastasis (arrow) to an intramammary lymph node.
DISCUSSION
We set out to explore amino acid transport imaging using 18F-fluciclovine in primary breast lesions and correlate our findings to histologic and immunohistochemical characteristics of breast cancer. Our results demonstrate higher uptake in malignant than benign breast lesions, with similarly low uptake in benign and normal breast tissue across all time points. 18F-Fluciclovine activity also seemed to vary with histologic characteristics, hormone receptor status, and tumor grade, with higher uptake seen in triple-negative and NG3 breast cancers. 18F-Fluciclovine uptake had weak positive correlation with Ki-67 intensity. Within the context of the small patient sample in this exploratory study, 18F-fluciclovine PET visualized locoregional and distant spread except for hepatic metastasis secondary to physiologic hepatic uptake (11). In primary lesions, 18F-fluciclovine activity in IDC seemed higher than in ILC, but the opposite was present with locoregional nodes. In addition, 18F-fluciclovine activity was higher in ILC than 18F-FDG. Though these observations are based on limited data, further investigation of 18F-fluciclovine PET in relation to 18F-FDG PET and with a focus on lobular breast cancer seems warranted. Results of this exploratory trial can be used to guide further study design. Because lesion kinetics suggest that equilibrium is established quickly between malignant lesions and background structures, we believe that future trials should concentrate on the earlier time period after 18F-fluciclovine injection.
Other metabolic activity upregulated in breast cancer includes that of amino acids. The amino acid transporters, specifically LAT1, ASCT2, ATB0,+ SNAT1, and XCT, are overexpressed in breast cancer and reported to be associated with tumor growth, metastasis, and hormone receptor status (12,20–24). Our findings are similar to reports on amino acid PET imaging including l-[1-11C]tyrosine and l-[methyl-11C]methionine (MET), which reported higher uptake in malignant breast tumors than benign and background structures, with better tumor contrast on MET than 18F-FDG (25,26). Yet, these radiotracers also have drawbacks, including incorporation into metabolic pathways and the 20-min half-life of 11C necessitating proximity to a cyclotron. 18F-Fluciclovine is a nonmetabolized synthetic amino acid–based PET radiotracer transported via LAT1 and ASCT2, with the advantage of the 110-min half-life of 18F (11). We have previously reported on whole-body biodistribution of 18F-fluciclovine and its potential utility in breast cancer (11,17,27).
Though limited by small sample size, 18F-fluciclovine demonstrated the greatest uptake with metaplastic cancer, followed by IDC, then ILC, with significantly higher mean 18F-fluciclovine uptake in NG3 than in NG2 and NG1 breast cancers. There was also higher 18F-fluciclovine uptake in triple-negative than ER+/HER2− and HER2+ breast cancers. These findings are similar to those reported with 18F-FDG PET and indicate the more aggressive nature of triple-negative cancers (28,29). Shennan noted variation in expression of system L amino acid transporters in MDA-MB-231 (ER-negative [ER−]) and MCF-7 (ER+) breast cancer cell lines (30). Increased expression of ASCT2 has also been associated with prognostic indicators of breast cancer such as high-grade, ER−, PR−, triple-negative, and HER2+ breast cancers (23,31). We found significant but weak positive correlation between Ki-67 and 18F-fluciclovine uptake across time points. Our findings are similar to our prior report of weakly positive though nonsignificant correlation between Ki-67 and 18F-fluciclovine uptake in lung cancer (32). Similar findings have also been reported in a meta-analysis of Ki-67 and 18F-FDG in breast cancer (33).
Limitations of this study include the small sample size and limited distribution across molecular subtypes and Ki-67. Not all types of benign lesions such as fibroadenomas or fat necrosis were studied. Observations on the detection of metastatic disease or comparison to 18F-FDG PET are preliminary. This exploratory study was designed to concentrate on dynamic uptake characteristics in primary lesions, and only 1 imaging bed position was acquired. The use of a whole-body PET/CT device for study of primary breast lesions limits observation for small lesions and results in underestimation of SUV. Partial-volume correction was not done. Positron emission mammography would be useful for further study but is not widely available. Six patients underwent 18F-fluciclovine PET/CT after biopsy, but inflammation was likely mitigated because at least 8 d passed between the procedure and imaging. Though we reported SUVmax for target structures, overall similar conclusions were made using SUVmean and SUVpeak (Supplemental Figs. 1 and 2; Supplemental Table 1 [supplemental materials are available at http://jnm.snmjournals.org]).
CONCLUSION
In this exploratory study, 18F-fluciclovine had a significantly higher uptake in malignant than benign breast lesions and normal breast tissue. 18F-Fluciclovine activity seemed to vary with histologic and immunohistochemical characteristics of malignant breast tumors and demonstrated highest uptake in breast cancers, with poor prognostic factors. Further studies correlating whole exome RNA sequencing and amino acid transporter expression to 18F-fluciclovine uptake in breast lesions are ongoing. Larger trials to determine the potential clinical benefits of 18F-fluciclovine PET for staging, determination of therapy response, and diagnostic performance compared with conventional imaging and 18F-FDG PET are required.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. Mark M. Goodman and Emory University are entitled to royalties derived from the sale of products related to the research described in this manuscript. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. 18F-Fluciclovine cassettes were provided to Emory University by Blue Earth Diagnostics Ltd. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We acknowledge Bellamy Leah Madge, RN; Oyeladun Oyenuga, MD MPH; Oladunni Akin-Akintayo, MD MPH; Ibeanu Ijeoma, MD MPH; Fenton G. Ingram, RT(R), CNMT, PET; Seraphinah Lawal, RT(R), CNMT, PET; Adam Brown, RT(N), CNMT; Ronald J. Crowe, RPh, BCNP; and the cyclotron and synthesis team from the Emory University Center for Systems Imaging.
Footnotes
Published online Apr. 7, 2016.
- © 2016 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication December 16, 2015.
- Accepted for publication March 9, 2016.