Abstract
The overall median survival of patients with a malignant glioma is <1 y. Because malignant gliomas rarely metastasize outside the skull, locoregional treatment strategies, such as gene therapy, are under investigation. Recently, convection-enhanced delivery (CED) has been presented as a method to improve delivery of large molecules. The goal of this study was to evaluate whether CED improves intratumoral delivery of adenoviral vectors and compare it with single injection (SI) and multiple injection (4×, MI). Methods: A replication-deficient adenoviral vector encoding the herpes simplex virus thymidine kinase (HSV-tk) and the human somatostatin receptor subtype 2 (sst2) was administered into nude mice bearing subcutaneous U87 xenografts. Tumors were injected with 1.5 × 109 plaque-forming units of Ad5.tk.sstr by CED, SI, or MI. Three days later, [99mTc-N40–1,Asp0,Tyr3]octreotate (99mTc-Demotate 2) was injected intravenously to monitor the virus-induced sst2 expression. γ-Camera imaging was performed for in vivo imaging, and the tumor uptake of 99mTc-Demotate 2 was determined by γ-counter. Furthermore, the tumor was sectioned and ex vivo autoradiography was performed. After decay of radioactivity, adjacent sections were submitted to in vitro autoradiography with 125I-DOTA-Tyr3-octreotate, which was used to calculate the transduced areas. Results: Transfected xenograft tissues showed high sst2 expression and were clearly visualized with a γ-camera. Accumulation of radioactivity was 2-fold higher in the tumors that were injected with MI compared with CED and SI (P = 0.01). CED and SI resulted in equal uptake of radioactivity in the tumors. The measured areas of transduction in ex vivo and in vitro autoradiographs showed a high concordance (r2 = 0.89, P < 0.0001). The maximum area of transfection was significantly larger after MI than after CED (P < 0.05) or SI (P = 0.05). Also, the measured volume of distribution was twice as high after administration of Ad5.tk.sstr by MI (56.6 mm3) compared with SI (25.3 mm3) or CED (26.4 mm3). Conclusion: CED does not increase adenoviral vector distribution in a glioma xenograft model compared with SI. Therefore, in the clinic MI is probably the most effective delivery method for the large adenoviral particle (70 nm) in malignant gliomas.
The overall prognosis of patients with malignant gliomas is dismal, with a median survival of <1 y (1). Despite progress in neurosurgery, chemotherapy, and radiotherapy, the survival of these patients has not changed during the last several decades. Because of the poor prognosis of malignant gliomas, new treatment modalities are being developed. As malignant gliomas rarely metastasize outside the skull, some modalities focus on locoregional treatment strategies. Examples include local application of wafers impregnated with cytotoxic drugs, local application of targeted toxins, and intratumoral injection of viral vectors for gene therapy or virotherapy (2–4).
Adenoviral vectors are among the most promising gene delivery vehicles currently available for glioma therapy, because they can be produced in large quantities in high-titer batches and because relatively large segments of foreign DNA carrying therapeutic genes can be incorporated. Recently, a small randomized study clearly demonstrated the efficacy of an adenovirus serotype 5 (Ad5) carrying the herpes simplex virus thymidine kinase gene (HSV1-tk) in combination with ganciclovir in malignant glioma (5). Ad5-tk treatment significantly increased the mean survival from 39 to 71 wk (P < 0.01), indicating some efficacy of the treatment.
In spite of these promising results, 2 major hurdles have been identified that limit the efficacy of Ad5-based gene therapy in malignant gliomas. First, the coxsackie adenovirus receptor (CAR) is poorly expressed on primary glioma cells, resulting in low transduction efficiency compared with established cell lines (6). Various strategies have been developed to successfully retarget adenoviral vectors to other receptors that are expressed at higher levels on malignant glioma cells, such as integrins or CD46 (7,8). Another major obstacle is the limited tissue penetration of the virus after injection into glioma tissue. A carefully conducted clinical study demonstrated that the distribution of the vector into brain tissue is limited to an average 5 mm from the needle tract (9). One approach to improve tissue penetration is the development of conditionally replicative adenoviruses (CRAds) (10,11). The release of CRAd progeny by infected tumor cells provides a potential to amplify the oncolytic effect by lateral spread through solid tumors.
Another way to improve tissue penetration of the virus is the exploration of new delivery methods. Recently, convection-enhanced delivery (CED) was developed as a means to improve delivery of macromolecules throughout the brain (12–14). CED is based on continuous infusion of drugs via intracranial catheters, enabling convective distribution of high drug concentrations over large volumes of the target tissue (15). CED has been successfully applied in clinical glioma trials to administer large molecules, including immunotoxins (4,16). CED has also been used to deliver viral vectors, including adenovirus, to the brain. In experimental models, delivery of viral vectors by CED resulted in improved transduction of the brain (17–21).
In clinical gene therapy trials, the adenoviral vectors were administered into the gliomas by either a single injection (SI), through a catheter, or by multiple wound bed injection after reresection (2,5,9). The goal of this study was to evaluate whether CED improves intratumoral delivery of an adenoviral vector in a mouse xenograft glioma model. We compared the volume of tumor that was transduced by the adenoviral vector after CED with the volumes obtained by SI and multiple injection (4×, MI) of the same amount of virus.
To monitor the distribution of the adenoviral vector, we used the human somatostatin receptor subtype 2 (sst2) gene in combination with the radiolabeled tracer [99mTc-N40–1,Asp0,Tyr3]Octreotate (99mTc-Demotate 2), which has high selectivity and high affinity for the sst2 receptor (22). We found that radioactive uptake and volume of distribution after CED were comparable to SI. After MI, the transduced tumor volume and the maximum area of transduction were 2- to 3-fold increased compared with both SI and CED.
MATERIALS AND METHODS
Cell Culture
The U87MG human glioblastoma multiforme (GBM) cell line was obtained from the American Type Culture Collection; the U251 human GBM cell line was from Peter Körnblith (National Institutes of Health, Bethesda, MD), and the rat pancreatic tumor cell line CA20948 was available at our institution (23). U87MG cells were cultured in minimum essential medium (MEM) (Invitrogen) with 0.1 mmol/L nonessential amino acids, 2 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 1,500 mg/L sodium bicarbonate, 10% heat-inactivated (30 min, 56°C) fetal bovine serum (FBS) (Invitrogen), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). U251 was cultured in Dulbecco's modified Eagle medium (DMEM) (Invitrogen) containing 4,500 mg/L glucose, 580 mg/L l-glutamine, and 110 mg/L pyruvate with 10% FBS (Invitrogen), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). CA20948 was cultured in DMEM as described with 0.1 mg/L fungizone supplemented. Medium was changed twice a week. Cells were cultured at 37°C in a 5% CO2 atmosphere.
Adenoviral Vectors Ad5.tk.sstr and Ad5.CMV.nLacZ
Construction of Ad5.tk.sstr and Ad5.CMV.nLacZ has been described earlier (24,25). Briefly, Ad5.tk.sstr is a replication- incompetent adenoviral vector that contains the sst2 and the herpes simplex virus thymidine kinase (tk) genes in the deleted E1 region. Both genes are regulated by separate immediate early cytomegalovirus (CMV) promoters. The E1-deleted Ad5.CMV.nLacZ vector contains the β-galactosidase (nLacZ) driven by a CMV promoter. Both vectors were propagated to high titers on PER.C6 cells, CsCl gradient purified, dialyzed, and stored at −80°C in sucrose buffer (140 mmol/L NaCl, 5 mmol/L Na2HPO4, 1.5 mmol/L KH2PO4, 20 mmol/L MgCl2, and 5% sucrose). All batches were screened for replication-competent adenovirus (RCA) (26) and met the criterion of <1 plaque-forming unit (pfu) of wtE1A promoter containing virus per 107 pfu. Titrations were performed on 911 cells and are presented as pfu/mL (27). The titers ranged from 6 × 1010 to 3 × 1011 pfu/mL.
Radiolabeling of Peptides
99mTc-Demotate 2 was provided by Dr. Theodosia Maina (Institute of Radioisotopes, Athens, Greece) and prepared as described earlier (22). Briefly, 20 μg Demotate 2 (10−3 mol/L), in 50 μL 0.5 mol/L phosphate buffer (pH 10.5), and 5 μL 0.1 mol/L Na3-citrate and 410 μL 99mTcO4 (Ultratechnekow generator; Mallinckrodt Medical) were mixed, and the reaction was started by the addition of 20 μg SnCl2 (2 mg SnCl2 per mL ethanol, freshly made) at room temperature. After 15 min, another 20 μg SnCl2 in ethanol were added and mixed. After 30 min, 8 μL of 1 mol/L HCl and 50 μL ethanol were added and the solution was sterilized by filtration through a Millex 0.22-μm GV filter (Millipore). The radiochemical purity was tested by high-performance liquid chromatography and was >90%. The mean specific activity of 99mTc-Demotate 2 was 40–200 MBq/μg.
DOTA-Tyr3-Octreotate was labeled with 125I as described earlier (28). The mean radiochemical purity was >90%. The mean specific activity of DOTA-125I-Tyr3-octreotate was 0.2 MBq/mmol. Octreotide was supplied by Novartis. All chemicals used were purchased from Aldrich.
Adenoviral Stability
During CED, the adenoviral vector is exposed to room temperature for up to 3 h and to transport through Teflon (DuPont) tubing. To examine the effect of room temperature on adenoviral stability, we sampled aliquots of Ad5.CMV.nLacZ placed on the bench at room temperature every 30 min. To study the effect of passage through tubing, Ad5.CMV.nLacZ from the same batch was pushed (1 μL/min) through the same tubing used for CED experiments over a 3-h period and aliquots were sampled every 30 min. Infectivity of the aliquots was examined by infecting U251 cells and measuring β-galactosidase activity. U251 cells were plated into 24-well plates at a density of 2 × 105 cells per well in 1 mL DMEM containing 10% FBS and incubated overnight at 37°C. After changing the medium, the aliquots containing Ad5.CMV.nLacZ were added in triplicate at a concentration of 100 pfu per cell. The medium was changed 2 h after starting the infection. After 48 h of incubation at 37°C the medium was removed, the cells were washed with phosphate-buffered saline (PBS), and assayed for β-galactosidase activity by the Galacto-Light Plus system (Applied Biosystems) using a Packard Top Count NXT luminometer (PerkinElmer Life Sciences). The same experiments were repeated after the addition of bovine serum albumin (BSA) (New England Biolabs) at a concentration of 0.1 mg/mL.
Animal Experiments
All experimental protocols were approved by the Institutional Animal Care and Use Committee, in compliance with the Guide for the Care and Use of Laboratory Animals. Male NMRI nu/nu mice (Charles River), 5–6 wk of age, were purchased. Mice were maintained at 3 or 4 per cage and allowed access to food and water ad libitum. After 1 wk, 1 × 107 U87MG or CA20948 cells were inoculated subcutaneously into both flanks in 250 μL of Hank's balanced salt solution (Invitrogen). The tumor growth was assessed 3 times a week by measuring bidimensional diameters with calipers. The tumor volume was determined by using the simplified formula of a rotational ellipse (length × width2 × 0.5) (29). Anesthesia was induced with isoflurane in oxygen and nitrous oxygen. Body temperature was maintained at 37°C using a heat pad. When the tumors reached a volume of approximately 250 mm3, animals were injected with a total dose of 1.5 × 109 pfu of Ad5.tk.sstr or PBS into the tumor. We compared 3 delivery strategies: SI, (4×, MI), and CED. In SI experiments, the total viral dose was administered in 22 μL with 1 injection. In the MI experiments, the total viral dose was diluted in 50 μL and was administered by 4 separate injections into 4 different tumor quadrants. In CED, the viral dose was diluted in 180 μL and was infused at 1 μL/min for 180 min. The continuous infusion was performed with a constant-flow pump (Harvard Apparatus). A 27-gauge hypodermic needle was connected to an air-tight 250-μL Hamilton syringe with 30 cm of Teflon tubing. For SI and MI, we used a 50-μL Hamilton syringe fitted with a 27-gauge needle. As a positive control we used mice bearing sst2-positive rat pancreatic CA20948 xenografts. Seventy-two hours after administration of the vector, 0.5 μg 99mTc-Demotate 2 was injected into the dorsal vein of the penis in a total volume of 200 μL. γ-Camera images were acquired for 10 min, 3.5 h after 99mTc-Demotate 2 injection, using a Rota II γ-camera system (Siemens) equipped with a low-energy collimator. The animals were sacrificed 4 h after 99mTc-Demotate 2 injection. The tumors were taken out and weighed. After determining the radioactivity in a LKB-1282-Compugammasystem (Perkin Elmer) γ-counter, the tumors were frozen in Tissue-Tek (Sakura). For each adenovirus-injected tumor, the total background activity was calculated on the basis of the means of the PBS-injected U87MG tumors (percentage injected dose per gram of tissue [%ID/g]). After subtracting the background activity, the adenoviral vector–induced radioactivity was expressed as percentage of injected dose (%ID) per tumor.
Autoradiography
The presence of radioactivity in tissue sections was detected by ex vivo and in vitro autoradiography. Tumors were embedded in TissueTek and processed for cryosectioning. Tissue sections (10 μm) were obtained at 0.3-mm intervals throughout the tumor and mounted on SuperFrost Plus slides (Menzel). For ex vivo autoradiography, sections were exposed to phosphor screens (Packard Instruments) for 2 d. The screens were analyzed using a Cyclone Phosphor Imager (Packard Instruments) and a computer-assisted OptiQuant 03.00 image-processing system (Packard Instruments). In vitro autoradiography was performed on the tumor sections after decay of 99mTc-Demotate 2. Tissue sections (10 μm) were mounted on glass slides and stored at −20°C for at least 1 d to improve adhesion of the tissue to the slide. The sections were preincubated for 10 min at 4°C, using buffer A, containing 167 mmol/L Tris-HCl (pH 7.7), 5 mmol/L MgCl2, and 0.25% BSA. The sections were then incubated for 60 min in the same buffer, in the presence of 10−10 mol/L 125I-DOTA-Tyr3-octreotate (DOTA is 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid), 1% BSA, and 40 μg/mL Bacitracin (Sigma-Aldrich). The sections were then washed twice for 5 min in buffer A and once in buffer A without BSA. After a short wash with distilled water, the sections were dried and exposed to phosphor image screens for 1 d. Nonspecific binding was determined in an adjacent section in the presence of 10−6 mol/L octreotide. Sections of rat brain and CA20948 tumor served as positive controls.
Volume of Adenoviral Vector Distribution
We used the in vitro autoradiography slides to calculate the volume of tumor transduced by the adenoviral vector. On the sections, we defined the boundaries of radioactivity by setting a threshold of 5 times background activity. We then used Scion Image software (Scion) to calculate the area of radioactivity. Then, we plotted the areas of all adjacent slides and determined the area under the curve expressing the volume of distribution using GraphPad Prism version 4.0 software (GraphPad Software, Inc.).
Statistical Analysis
Data were analyzed using GraphPad Prism software. All tests (t test, ANOVA) were 2-sided and P < 0.05 was considered statistically significant.
RESULTS
Adenoviral Stability During CED
Delivery by CED exposes the adenoviral vector to 3 h of room temperature and to passage through the tubing. To determine the effect of these factors on adenoviral stability, we simulated a 3-h CED experiment on the bench top. After exposure to 3 h of room temperature, the infectivity of the vector dropped from 2.3 × 106 relative light units (RLU) to 1.3 × 106 RLU without passage through the tubing and to 1.4 × 106 RLU after additional exposure to the tubing (Fig. 1). The decline of infectivity over time was significant in both instances (P < 0.01) but was primarily due to a drop in the first 30 min. Clearly, passage through the tubing did not negatively affect the infectivity of the vector. We then examined the effect of addition of BSA on stability. After 3 h on the bench top at room temperature, the infectivity remained stable at 2.0 × 106 RLU. After a 3-h exposure followed by passage through the tubing, the infectivity of the vector dropped from 2.0 × 106 to 1.5 × 106 RLU (P = 0.09). Because addition of BSA did not significantly increase vector stability during simulation of CED on the bench top, in vivo experiments were performed without BSA.
Adenoviral vector stability at room temperature after passage through Teflon tubing. Aliquots of Ad5.CMV.nLacZ, stored at room temperature (RT), were sampled every 30 min over a 3-h period. In parallel, aliquots from the same batch were collected after transport through Teflon tubing (1 μL/min). U251 cells were incubated with aliquots (100 pfu/cell), and β-galactosidase activity was measured 2 d later. β-Galactosidase activity decreased over time but was not affected by transport through tubing. Error bars represent SD.
γ-Camera Images After CED, SI, and MI of Ad5.tk.sstr
Animals carrying established bilateral subcutaneous U87MG tumors were injected with 1.5 × 109 pfu of Ad5.tk.sstr or PBS. Three different delivery strategies were used: SI, (4×, MI), and CED. Three days after the injection of the viral vector, the radioactive tracer 99mTc-Demotate 2 was injected intravenously and the animals were imaged. All Ad5.tk.sstr–injected tumors were readily visualized with radioactive 99mTc-Demotate 2 with a γ-camera (Fig. 2). In addition, a stronger signal was visible in tumors injected with MI than that in tumors treated with SI or CED. These findings indicate the feasibility of in vivo imaging using the Ad5.tk.sstr/99mTc-Demotate 2 system.
γ-Camera images of NMRI nu/nu mice. Mice bearing U87MG xenografts 3.5 h after intravenous administration of 0.5 μg 99mTc-Demotate 2 (100 MBq). Three days before imaging, tumors had been injected with PBS (left) or 1.5 × 109 pfu of Ad5.tk.sstr administered with CED, SI, or MI (4x, MI). Both tumors in each animal received the same treatment. Images are representative and demonstrate that MI results in better tracer uptake than CED and SI. B = bladder; K = kidney; T = tumor.
Radioactivity Accumulation in Tumor
After imaging, the animals were sacrificed and the radioactivity in the tumors was measured (Table 1). The accumulation of radioactivity (%ID) was significantly higher in the tumors that were injected with MI compared with CED and SI (P = 0.01), confirming the γ-camera results. CED and SI resulted in equal uptake of radioactivity in the tumors.
Virus-Induced Tumor Uptake of 99mTc-Demotate 2 in Xenograft-Bearing Mice After Ad5.tk.sstr Administration by CED, SI, or MI
Autoradiography and Quantitation of Vector Distribution
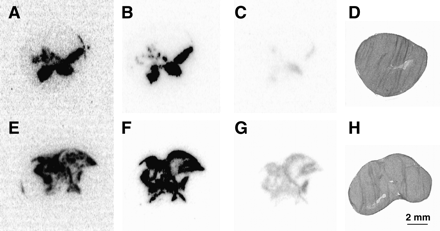
After sectioning, U87MG xenografts were submitted to autoradiography (Fig. 3). Ex vivo autoradiographs visualized transduced areas of the tumors (Figs. 3A and 3E). The representative autoradiographs clearly showed a larger area of radioactivity in the section of the tumor treated with MI (Fig. 3E) than that treated with SI (Fig. 3A). After decay of 99mTc-Demotate 2, in vitro autoradiographic studies were performed with 125I-DOTA-Tyr3-octreotate and showed areas of transduction similar to the ex vivo autoradiographs (Figs. 3B and 3F). Adding excess unlabeled octreotide completely blocked uptake of radioactivity, indicating the specificity of the observed binding of 125I-DOTA-Tyr3-octreotate to the transduced sst2 receptor (Figs. 3C and 3G). The area of transduction was calculated for each section using Scion Image software. The area of transduction calculated on the in vitro autoradiographs correlated significantly with the area calculated on the ex vivo autoradiograph of the same section (r2 = 0.89, P < 0.0001). Because the in vitro autoradiographs can be obtained from a large number of sections at the same time, we used this method to determine the maximum area and volume of transduction for all tumors. First, we determined for each tumor the maximum area of transduction on a single section for each delivery method (Fig. 4A). The maximum area of transduction was significantly larger after MI than after CED (P < 0.05) or SI (P = 0.05). Then, we plotted the areas of all adjacent slides and calculated the area under the curve representing the volume of distribution. Figure 4B presents the volumes of distribution obtained with the 3 different delivery techniques. The volume of distribution was twice as high after administration of Ad5.tk.sstr by MI (56.6 mm3) compared with SI (25.3 mm3) or CED (26.4 mm3).
Autoradiographs of Ad5.tk.sstr–injected U87MG xenografts by single injection (A–D) or by multiple injection (E–H). Ex vivo autoradiographs were performed 4 h after injection of 99mTc-Demotate 2 (A and E). In vitro autoradiographs were performed on adjacent slides with 125I-DOTA-Tyr3-octreotate (B and F). Binding was displaced by excess unlabeled octreotide (C and G). Adjacent tumor sections were visualized with hematoxylin–eosin staining (D and H). Representative sections demonstrate that the area of viral distribution is larger in sections from tumors injected with MI compared with SI.
Quantitation of vector distribution. Area of vector distribution was calculated using serial in vitro autoradiography sections incubated with 125I-DOTA-Tyr3-octreotate. (A) In each tumor, the section with the largest area was selected. Maximum area of vector distribution depends significantly on method of vector delivery (MI vs. CED, P < 0.05; MI vs. SI, P = 0.05; CED vs. SI, P = 0.8). (B) To calculate volume of vector distribution, all radioactive areas of consecutive sections were plotted and the area under the curve was calculated. Data are mean ± SEM, 8 tumors in each group.
DISCUSSION
The local pattern of recurrence and absence of metastasis outside the central nervous system make malignant gliomas suitable candidates for locoregional treatment strategies, including gene therapy. CED was recently developed to increase the volume of distribution of large molecules through the brain (12–14,21). In this study, we applied CED in an attempt to improve the local delivery of adenoviral vectors. We compared CED with SI and (4×, MI) of a replication-deficient adenoviral vector in a subcutaneous mouse xenograft tumor model. To monitor the distribution of the virus, we inserted the gene encoding the human sst2. Expression of sst2 was visualized in vivo by intravenous injection of 99mTc-Demotate 2 followed by γ-camera imaging, and the accumulation of radioactivity was quantitated with a γ-counter. The volume and maximum area of transduction of the tumor were taken as a measure of in vivo vector distribution and was measured using in vitro autoradiography. Before comparing CED with MI and SI, we investigated the adenoviral stability and recovery from the CED infusion device. Sanftner et al. (21) found up to 90% absorption of an adeno-associated virus, determined by quantitative polymerase chain reaction, to the device depending on the tubing used. These authors observed excellent vector recovery when using fused silica and silicon tubing. In our adenoviral test setting, the Teflon tubing did not affect adenoviral infectivity, indicating good vector recovery. However, exposure of the vector for 3 h at room temperature resulted in a 43% decline of infectivity. The decline in infectivity by exposure to room temperature was most prominent in the first 30 min and was not prevented by addition of BSA.
Direct comparison of CED with SI and MI demonstrated that both accumulation of radioactivity and the largest area of transduction on a single section were significantly larger after MI than after CED or SI. Also, the volume of transduction was increased 2-fold. However, CED did not increase the maximum area or the volume of tumor transduction compared with SI. Lack of distribution enhancement by CED compared with SI could be attributed to the flow, time, and volume settings of CED; the size of the adenoviral particle; or the interaction between the adenovirus and the tissue.
For optimal CED of drugs, several relevant variables have been identified, including catheter size, flow rate, molecular weight of the drug, and tissue characteristics (30,31). Backflow of the infused drugs is dependent on catheter size and flow rate (30), which should not exceed 1 μL/min (19). We used the recommended maximum flow rate and did not observe backflow. Furthermore, increased molecular weight and particle size decrease delivery (12,14,32,33). Betz et al. (18) applied CED to deliver a replication-defective adenoviral vector (70–100 nm) (34) to rat brain and studied various infusion parameters. Variations in flow rate, concentration, and infectious titer resulted in a volume of adenoviral transduction ranging from 4 to 27 mm3 for 0.3–9 × 109 virus particles. MacKay et al. (32) performed CED experiments using different-sized liposomes (40–200 nm in diameter) and reported a reduction in brain penetration with increase in size. Kroll et al. (35) reported that increasing the dose of monocrystalline iron oxide nanocompounds (viral-sized agent) rather than convection might result in the best volume of transduction. On the other hand, Brust et al. (19) showed that infusion of 1 μL/min over a longer period of time (2.5 h) is more effective than infusion of smaller volumes with the same dose of adenovirus. Although we used the same infusion rate and a volume of 180 μL, we found comparable levels of radioactive accumulation between SI and CED, indicating that in our setting the increase of injected volume did not result in an improved volume of distribution.
We used the stt2 marker gene to visualize adenoviral vector distribution in vivo and ex vivo. Recently, Maina et al. (22,36) described new somatostatin analogs, 99mTc-Demotate 1 and 99mTc-Demotate 2, for the visualization of the somatostatin receptor in endocrine tumors. At present, [111In-DTPA0]octreotide (DTPA = diethylenetriaminepentaacetic acid) (111In-OctreoScan; Mallinckrodt) is the standard substrate for diagnostic imaging and staging of sst2-expressing tumors. However, somatostatin analogs based on 99mTc are promising alternatives because of optimal nuclear characteristics, low cost, and easy availability via commercial 99Mo/99mTc generators (22). In this study, we found high 99mTc-Demotate 2 uptake in infected U87MG xenografts by in vivo γ-camera imaging and ex vivo autoradiography. The high, significant correlation between the ex vivo and in vitro autoradiographs indicates that the intravenously injected 99mTc-Demotate 2 efficiently permeates the entire tumor.
Our results showed no significant difference in adenoviral vector distribution between CED and SI but, conversely, showed an increase in radioactive uptake and volume of distribution in MI. For that reason, in the clinic, MI is probably the most effective delivery method for the large adenoviral particle (70 nm) in malignant gliomas. Several clinical studies have demonstrated that administration of adenoviral vectors through multiple injections into the brain is probably safe, although silent hemorrhages and transient neurologic deficits have been described (2,9). Our study shows that even small experimental tumors are not entirely transduced after MI. Additional strategies such as radionuclide therapy with, for example, 177Lu- and 90Y-labeled somatostatin analogs (37) or the HSV-tk/ganciclovir “bystander effect” (38,39) or using (conditional) adenoviral replication (10,11) will be required to obtain eradication of experimental tumors or clinically relevant tumor responses.
CONCLUSION
Our study showed that CED did not increase adenoviral vector distribution in a glioma xenograft model compared with SI. Therefore, in the clinic, MI is probably the most effective method to deliver the large adenoviral particle into (brain) tumors. Furthermore, 99mTc-Demotate 2 seems a good candidate to image and quantitate the distribution and expression level of the sst2 marker.
Acknowledgments
We thank Mark Rodijk, Monique de Visser, Marleen Melis, and Wout Breeman for technical support. We also thank Theodosia Maina (Institute of Radioisotopes, Athens, Greece) for providing Demotate 2. Support was provided by the Dutch Cancer Society, Koningin Wilhelmina Fonds (grant DDHK 2001-2459), and an Erasmus Medical Center translational research grant.
Footnotes
-
COPYRIGHT © 2006 by the Society of Nuclear Medicine, Inc.
References
- Received for publication December 13, 2005.
- Accepted for publication June 12, 2006.