Visual Abstract
Abstract
The radiation dose to the kidneys should be monitored in prostate cancer patients treated with radioligand therapy (RLT) targeting the prostate-specific membrane antigen (PSMA). We analyzed whether pretherapeutic kidney function is predictive of subsequent kidney dose and to what extent the cumulative kidney dose at the end of multiple therapy cycles can be predicted from a dosimetry based on the first cycle. Methods: Data of 59 patients treated with at least 2 cycles of 177Lu-PSMA-617 (PSMA RLT) were analyzed. Treatment (median, 6 GBq/cycle) was performed at 6- to 8-wk intervals, accompanied by voxel-based 3-dimensional dosimetry (measured kidney dose) with SPECT/CT on each of days 0–3 and once during days 6–9. Pretherapeutic kidney function (estimated glomerular filtration rate, mercaptoacetyltriglycine clearance) was correlated to the kidney doses. Cumulative kidney doses at the end of treatment were compared with a dose estimated from the population-based mean kidney dose, individual first-cycle kidney dose, and mean kidney doses of cycles 1, 3, and 5 per administered activity. Results: In total, 176 PSMA RLT cycles were performed, with a median of 3 cycles per patient. The average kidney dose per administered activity of all 176 cycles was 0.67 ± 0.24 Gy/GBq (range, 0.21–1.60 Gy/GBq). Mercaptoacetyltriglycine clearance and estimated glomerular filtration rate were no reliable predictors of subsequent absorbed kidney dose and showed only small effect sizes (R2 = 0.080 and 0.014 [P = 0.039 and 0.375], respectively). All simplified estimations of cumulative kidney dose correlated significantly (P < 0.001) with measured kidney doses: estimations based on the individual first-cycle dose were more accurate than the use of the population-based average kidney dose (R2 = 0.853 vs. 0.560). Dose estimation was best when the doses of cycles 3 and 5 were included as well (R2 = 0.960). Conclusion: Pretherapeutic renal function was not predictive of subsequent kidney dose during therapy. Extrapolation of individual data from dosimetry of the first cycle was highly predictive of the cumulative kidney dose at the end of treatment. This prediction was further improved by the integration of dose information from every other cycle. In any case, because of a high interindividual variance, an individual dosimetry is advisable.
Prostate-specific membrane antigen (PSMA) is frequently overexpressed in prostate cancer. Aside from imaging with PET with ligands targeting this antigen, 177Lu-based radioligand therapies (PSMA RLTs) are an emerging and promising treatment option in patients with metastatic castration-resistant prostate cancer (1). The potential of PSMA RLT has been demonstrated in recent phase II trials (2–4), and the effectiveness is currently under investigation in a multicenter phase III trial (NCT03511664).
Although PSMA RLT is generally well tolerated and shows only mild side effects, the bone marrow, salivary glands, and kidneys are considered to be potentially dose-limiting organs (5). In this respect, PSMA RLT shares similarities with peptide-receptor radionuclide therapy (PRRT) for neuroendocrine tumors, which also show an overall good tolerability, with renal and hematopoietic toxicity being the main side effects. As the kidney dose has been a major concern for PRRT, various protocols for nephroprotection by coinfusion of amino acids have been developed over the years (6). Traditionally, a tolerance dose of 23 Gy for the kidneys is assumed in PRRT, based on external-beam radiation therapy data (7). As a consequence, meticulous renal dosimetry is recommended in RLTs and should be thoroughly integrated into treatment protocols (8).
Although the kidney dose in 177Lu-based PSMA RLT is in a range similar to that when using PRRT with 177Lu-DOTATATE, no protocol for nephroprotection has been established yet. Especially, there is no evidence that an amino acid coinfusion results in a lower radiation exposure to the kidneys. On the basis of more recent data on 177Lu-based therapies, a higher renal tolerability of up to a 40-Gy cumulative kidney dose is assumed in PSMA RLT in the absence of risk factors, also taking prognostic aspects of the treated patient into account (5).
To accurately assess kidney doses in PSMA RLT, various procedures for dosimetry have been developed, ranging from simple planar imaging (9) to more complex SPECT/CT-based protocols (10). In this study, the kidney dose per cycle was determined on the basis of a dosimetry protocol that includes 5 intratherapeutically acquired SPECT/CT scans. Using these data, we assessed whether the cumulative kidney dose at the end of multiple therapy cycles can reliably be predicted from the dosimetry of the first therapy cycle only.
MATERIALS AND METHODS
Patients
In this retrospective analysis, data of patients with metastasized castration-resistant prostate cancer who had been treated with 177Lu-labeled PSMA-617 between July 2015 and July 2020 were analyzed. Patients were eligible for this analysis if 2 or more cycles of PSMA RLT had been performed and complete 3-dimensional (3D) SPECT/CT dosimetry data were available, including a late SPECT/CT image from at least 6 d after injection.
Treatment eligibility and pretherapeutic examinations had been done in accordance with the recommendations of the German Society of Nuclear Medicine (11). Mercaptoacetyltriglycine (MAG3) renal scintigraphy was used to determine pretherapeutic MAG3 clearance, especially to exclude active ureter obstruction. Patients with proven, treated previous obstruction and an inconspicuous MAG3 scan were eligible for therapy.
According to the aforementioned guideline, a standard activity of 6 GBq of 177Lu-DOTA-PSMA-617 was applied. A reduced activity of 4 GBq was used only in cases of reduced bone marrow function or strongly impaired renal function.
The institutional review board (vote no. 326/18) approved this study, and all subjects gave written informed consent.
Synthesis of 177Lu-DOTA-PSMA-617
177Lu-DOTA-PSMA-617 was produced in compliance with good manufacturing practices using an automated radiosynthesis device (Modular-Lab PharmTracer) with low-bioburden single-use cassettes. The commercially available precursor (ABX) and reagents were prepared and sampled according to standard operating procedures. No-carrier-added 177LuCl3 was purchased from ITM, and the cassettes were supplied by Eckert and Ziegler Eurotope GmbH. Before the synthesis was started, 177LuCl3 (∼8 GBq), ammonium acetate buffer (0.5 M, pH 5.4), 50% ethanol, and isotonic saline vials were connected to the cassette. The Sep-Pak Light C-18 cartridge (Waters) was preconditioned with 4 mL of 50% ethanol and 6 mL of isotonic saline. The synthesis was started by transferring 177LuCl3 (∼8 GBq) into the reaction vessel preloaded with 70 μg (67 nmol) of DOTA-PSMA-617 and 100 μL of ethanol to prevent radiolysis. The ammonium acetate buffer (700 μL) was transferred through the radioactive vial into the reaction vessel. The radiosynthesis was performed at 75°C for 40 min in ammonium acetate buffer. The mixture was subsequently passed through the preconditioned Sep-Pak Light C-18 cartridge and washed with isotonic saline. The final product was eluted with 50% ethanol, diluted with isotonic saline and passed through a 0.22-μm sterile membrane filter into a presterilized product vial prefilled with 100–200 μL of Ditripentat-Heyl (diethylenetriaminepentaacetic acid, solution for injection). The quality control was conducted in adherence with European Pharmacopeia standards, including filter integrity and pH testing, limulus amebocyte lysate testing, radionuclide identity testing, and purity testing by determining the half-life and energy spectrum. Chemical and radiochemical purity (≥97%) were identified by radio-high-performance liquid chromatography, and the residual solvent was identified by gas chromatography. Finally, after release, a sample of the product formulation was tested for sterility by an independent institution (Biochem) according to the recommendations of the European Pharmacopoeia and U.S. Pharmacopoeia using the direct inoculation method.
SPECT/CT Imaging and Dosimetry
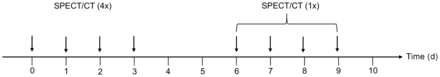
At each therapy cycle, imaging for dosimetry was performed on days 0–3, consisting of planar whole-body scans and abdominal SPECT/CT (including kidneys, liver, and spleen) at 1, 24, 48, and 72 h after injection. Moreover, 1 late SPECT/CT scan was acquired on an outpatient basis in the following week on day 6, 7, 8, or 9 after injection (Fig. 1).
Schematic overview of SPECT/CT measurements performed on days 0–3 (on inpatient basis) and on days 6, 7, 8, or 9 (on outpatient basis).
All acquisitions were performed on a SPECT/CT scanner (BrightView XCT; Philips Healthcare) equipped with medium-energy general-purpose collimators. Measurements were done with an energy window of ±10% around the 208-keV peak. SPECT was measured with 40 projections per head on a body-contour trajectory with a 128 × 128 matrix and a 20-s acquisition duration per projection. Attenuation correction was based on a cone-beam CT scan (30 mAs at 120 kV); SPECT was reconstructed iteratively with the ordered-subsets expectation maximization algorithm (4 iterations and 16 subsets; postreconstruction filter, Butterworth; cutoff, 0.4; order, 1.4). Whole-body scans were performed with a velocity of 20 cm/min and an imaging matrix of 256 × 1,024 to document the tracer distribution.
The SPECT/CT system was calibrated for 177Lu by phantom measurements with a National Electrical Manufacturers Association image-quality phantom using the aforementioned imaging protocol. The phantom body was filled with water, and two 500-mL volumes simulating kidneys were inserted, one filled with 100 MBq of 177Lu and the other with 200 MBq of 177Lu. Calibration measurements were done 4 times, at an interval of every other day. The resulting calibration factor was 9.9 ± 0.4 cps/MBq. 3D dose maps were calculated using STRATOS, which is part of the IMALYTICS Research Workstation (Philips Technology). The software package is based on the MIRD formalism for voxel-based dose calculation by voxel S values (12). The original SPECT images of each cycle were coregistered to the CT portion of the last scan in STRATOS and resampled to a voxel size of 4.42 × 4.42 × 4.42 mm in accordance with STRATOS’ voxel S-value sizes. The integral of the time–activity curve for each image voxel was calculated by the trapezoidal integration method until the last imaging time points, followed by an exponential tail fit using the physical half-life of 177Lu. Because of the late time point of SPECT/CT after 6–9 d, the dose contribution of the tail fit is very low and there is only a slight difference between the use of the physical half-life or the individual effective half-life.
The software package Rover (ABX) was used for kidney segmentation and 3D dose-map analysis (13).
In accordance with current guidelines (5), pretherapeutic kidney function before the first cycle was assessed using the estimated glomerular filtration rate according to the Chronic Kidney Disease Epidemiology Collaboration (14) (eGFRt1) and MAG3 clearance derived from renal scintigraphy performed before therapy in order to rule out obstructions. Estimated glomerular filtration rate was again determined approximately 2 wk before the third cycle (eGFRt2).
Statistics
For all patients and all cycles, the absorbed doses by the kidneys were calculated using voxel-based 3D dosimetry (measured kidney dose).
eGFRt1 and MAG3 clearance were correlated with the respective kidney dose of the first cycle for every patient, and if applicable, eGFRt2 was correlated to the third-cycle dose.
In all patients, the measured cumulative kidney dose was correlated with the estimated cumulative dose, based on our cohort’s mean kidney dose per administered activity (Gy/GBq) and on the individually calculated first-cycle kidney dose per administered activity. Moreover, to account for potential changes during therapy in patients receiving 4 or more therapy cycles, the extrapolation of the cumulative dose at the end of the treatment was done with the individual kidney dose per administered activity of every other cycle. In this case, the kidney dose of even-numbered cycles (cycles 2, 4, and 6) was estimated from the measured kidney dose of the therapy cycles taking place immediately beforehand (cycles 1, 3, and 5, respectively).
Correlations were based on linear regression using ANOVA for significance analysis. Paired t tests were used for comparisons of eGFRt1/t2 and kidney doses at different cycles. All analyses were performed using IBM SPSS statistics software, version 27. Arithmetic mean values were calculated from the individual measurements and expressed at a precision of 1 SD (mean ± SD).
RESULTS
Patient Treatment and Measured Kidney Doses
The data of 59 patients (aged 72.8 ± 8.5 y; median, 74.6 y) with advanced metastatic castration-resistant prostate cancer were eligible for analysis. The patients had received a median of 3 cycles of 177Lu-PSMA-617 (2 cycles, n = 28; 3 cycles, n = 11; 4 cycles, n = 16; 5 cycles, n = 1; 6 cycles, n = 3) at 6- to 8-wk intervals (total, 176 cycles). Average activity per cycle over all patients and cycles was 5.7 ± 0.8 GBq (median, 6.0 GBq) of 177Lu-PSMA-617 (cycle 1, 5.6 ± 0.9 GBq; cycle 2, 5.8 ± 0.7 GBq; cycle 3, 5.6 ± 1.0 GBq; cycle 4, 6.0 ± 0.2 GBq; cycle 5, 5.9 ± 0.3 GBq; and cycle 6, 5.9 ± 0.4 GBq). The cumulative measured kidney doses in all 59 patients after PSMA RLT ranged from 3.4 to 25.3 Gy. Average kidney dose per administered activity over all patients and cycles (n = 176) was 0.67 ± 0.24 Gy/GBq (range, 0.21–1.60 Gy/GBq). The respective kidney dose for each cycle is shown in Table 1. Average kidney doses per cycle did not differ significantly (P = 0.217; Fig. 2). Details on kidney doses depending on the number of cycles administered can be found in Supplemental Table 1 (total kidney dose) and Supplemental Table 2 (left and right kidney separately assessed) (supplemental materials are available at http://jnm.snmjournals.org).
Kidney Dose per Administered Activity at Each Cycle
Comparing kidney dose per administered activity (Gy/GBq) distribution per cycle (59 patients); no significant changes in any of 6 cycles were observed (P = 0.217).
Renal Function and Kidney Dose
The eGFRt1 ranged from 29.4 to 116.7 mL/min/1.73 m2 (76.5 ± 14.4 mL/min/1.73 m2). According to Kidney Disease Improving Global Outcomes (KDIGO) criteria (15), 13 patients presented with normal (KDIGO G1), 39 with mildly decreased (KDIGO G2), and 5 with a mildly to moderately decreased (KDIGO G3a) kidney function. One patient each presented with moderately to severely decreased (KDIGO G3b) and severely decreased (KDIGO G4) kidney function. MAG3 clearance ranged from 115 to 307 mL/min/1.73 m2 (202.8 ± 28.3 mL/min/1.73 m2) and correlated poorly with eGFRt1 (R2 = 0.167, P = 0.002). The kidney dose per administered activity (Gy/GB) observed after the first cycle correlated neither with eGFRt1 (R2 = 0.014, P = 0.375) nor with MAG3 clearance (R2 = 0.080, P = 0.039), with small effects of determination only. Similarly, the kidney dose per administered activity (Gy/GBq) of the first cycle did not correlate (R2 < 0.001, P = 0.85) with the amount of activity used (2.09–6.47 GBq). In particular, the 2 patients with more severely reduced kidney function did not receive higher kidney doses per gigabecquerel than did the other patients (0.69 and 0.48 Gy/GBq for KDIGO G3b and KDIGO G4, respectively). In 31 patients, eGFRt2 was determined; it ranged from 41.5 to 95.80 mL/min/1.73 m2 (72.3 ± 17.0 mL/min/1.73 m2). There was no correlation between eGFRt2 and the kidney dose (Gy/GBq) of the third cycle (R2 = 0.001, P = 0.993), and no significant change between eGFRt1 and eGFRt2 was observed (P = 0.96).
Predicted and Measured Kidney Doses
In all 59 patients, correlations between the measured and estimated cumulative kidney dose at the end of treatment were significant (P < 0.001). However, the use of the population-based mean kidney dose of 0.67 Gy/GBq for the prediction of the kidney doses at the end of treatment resulted in a greater variance (R2 = 0.560) than did the use of the individual first-cycle dose per administered activity (Gy/GBq) (R2 = 0.853). As expected, the approach using an individual dosimetry at every second cycle resulted in the best prediction (R2 = 0.960; Fig. 3).
Correlations of estimated and measured cumulative kidney dose in all 59 patients based on 3 models. The good correlation and coefficient of determination that were seen when extrapolation was done using individual dose per administered activity from first-cycle dosimetry (A) can be further improved when data of cycles 3 and 5 are also considered (B). In contrast, poorest coefficient of determination was observed using population-based average kidney dose only (C), in which estimation resulted in systematic dose underestimation, as can be seen in slopes.
Twenty patients received 4 or more cycles. Using the same dose estimation methods in this subgroup, the use of the mean kidney dose of 0.67 Gy/GBq did not result in a meaningful prediction (R2 = 0.166, P = 0.074). In contrast, the individual-based dosimetry estimations still correlated significantly with the measured cumulative kidney dose (P < 0.001). Dosimetry relying only on the first cycle showed a lower coefficient of determination than the approach using every other cycle (R2 = 0.630 vs. R2 = 0.947; Fig. 4).
Correlation of estimated and measured cumulative kidney dose in 20 patients receiving 4 or more therapy cycles based on dosimetry of cycle 1 only (red) and cycles 1, 3, and 5 (blue). Use of individual average kidney dose from every second cycle greatly improves associated coefficient of determination.
DISCUSSION
In the present study, the average kidney dose per administered activity was 0.67 ± 0.24 Gy/GBq of activity when performing a treatment with 6.0 GBq of 177Lu-PSMA-617. In comparison to the kidney doses reported in the European Association of Nuclear Medicine procedure guideline for PSMA-based RLT (5), this dose average is in the upper range of the reported dose of 0.4 ± 0.2 to 0.8 ± 0.3 Gy/GBq. This finding is not surprising, as we used recommended late-time-point measurements in the week after therapy (16) to avoid dose underestimation. In that sense, our results were consistent with the results of SPECT/CT-based dosimetry protocols also using late time points (5).
In accordance with the kidney dosimetry results reported by Okamoto et al. (9), who also included the important late time points in their dose calculations, we observed an intraindividually relatively constant development of kidney dose in our patients using fixed activities. Especially in responders to therapy, we did not observe postulated tumor sink effects (17), such as a reciprocal increase in intraindividual kidney dose per cycle due to decreasing tumor burden.
With a state-of-the-art multi-SPECT/CT 3D dosimetry approach, the high coefficients of determination in cumulative kidney dose based on personalized dosimetry show the feasibility of a linear extrapolation of the individual kidney dose within the bounds of a rigid treatment setting (i.e., activity and time intervals). Our data suggest that an individual dosimetry of the first cycle sufficiently predicts the cumulated kidney dose at the end of treatment. Obviously, we see a higher coefficient of determination for the estimated cumulative kidney dose when also including dosimetry data from cycles 3 and 5 (if applicable). Considerations comparable to our results in the prediction of kidney dose have been reported for PRRT in neuroendocrine tumors based on the dosimetry of the first 2 cycles (18). In addition to these dose prediction approaches, measures such as a reduction in the number of CT or SPECT/CT acquisitions per cycle (18,19) have been suggested to simplify often-elaborate dosimetry protocols (20). These simplifications would not only free scanner and staff capacities at the nuclear medicine facility but also improve patient comfort (21,22). Considering this, our approach of a thorough dosimetry of the first cycle followed by extrapolation appears to be a viable option, again provided that crucial late measurement time points are also included in such protocols to avoid potential underestimation (16,23). However, especially when aiming at a more streamlined dosimetry or larger dosimetry intervals, the high interindividual variation of the resulting kidney dose after PSMA RLT (0.28–1.29 Gy/GBq at the first cycle in our cohort), likewise observed in PRRT (23,24), must be considered. The poor cumulative dose estimation using a population-based mean kidney dose only, especially if patients received 4 or more cycles, shows that a reliable individual dosimetry is essential. Accordingly, one should refrain from using average kidney dose values from the literature for the prediction.
Our results showed that the pretherapeutic renal function was not predictive of the subsequent kidney dose during therapy. In our cohort, 57 of 59 patients had normal or only slightly impaired renal function before PSMA RLT. Even taking the 2 patients with more severely impaired kidney function, KDIGO G3b and G4, into account, we observed neither an association between pretherapeutic kidney function (eGFRt1) and the first-cycle kidney dose nor an association between kidney function after 2 cycles (eGFRt2) and kidney dose at the third therapy cycle. Although the association between the kidney dose of the first cycle and MAG3 clearance was significant, the associated effect size was very small (R2 = 0.062). These observations imply that kidney function alone is not sufficient for the prediction of the resulting kidney dose.
Although the role of potential risk factors for kidney damage (25), such as age, arterial hypertension, or previous renal impairment, still remains to be determined, retrospective PSMA RLT series have reported mild renal toxicity of grade 1 or 2 only (25,26). Safe administration of PSMA RLT is also possible even in patients with only a single kidney (27). These observations were confirmed by the updated analysis of the prospective phase II 177Lu-PSMA trial indicating that approximately 4 cycles of RLT are well tolerated and that renal impairment is to be expected only after a higher number of cycles (3). Similarly, nephrotoxicity was not a major adverse event in the recently published phase II TheraP trial (4). These observations on renal tolerance in 177Lu-PSMA RLT are partly comparable to data on renal tolerance of 177Lu-DOTATATE, as the phase III NETTER-1 trial did not show higher-grade (3 or 4) renal toxicity during the median 14 mo of follow-up (28). Even secondary salvage PRRT is sufficiently tolerated by the kidneys, whereas high-grade hematotoxicity is a more relevant issue (29). In this aspect, hematotoxicity may also be a more relevant side effect in patients with renal impairment, as a slower renal excretion might result in a longer exposure of the bone marrow due to circulating radioligand.
Although long-term data on the renal safety of PSMA RLT are still warranted, observations from PRRT suggest that the development of renal impairment is gradual and occurs over many years (30). Considering the often poorer prognosis of advanced metastatic castration-resistant prostate cancer than of neuroendocrine tumor disease, the individual risk of actually experiencing late kidney damage also has to be taken critically into account (5). Additionally, the risk of premature discontinuation of therapy in PSMA RLT due to disease progression and development of resistance to therapy must be heeded. Only one third of our patients received 4 or more therapy cycles, and as a consequence, only 6 patients had a cumulated kidney dose that came close to or even slightly surpassed the aforementioned conservative threshold of 23 Gy. Thus, in our cohort with a limited number of patients, kidney dose was not a reason for discontinuation of therapy.
CONCLUSION
For a current standard PSMA RLT treatment protocol, the resulting kidney doses were independent from pretherapeutic kidney function. Because of the observed almost linear correlation between treatment activity and the cumulative kidney dose in individual patients, a prediction of the cumulative kidney dose with dosimetry results from only the first cycle seems to be feasible. On the basis of our findings for patients with more than 4 therapy cycles, we recommend that dedicated dosimetry be performed during every other therapy cycle; this schedule offers a good compromise between effort, patient comfort, and accuracy in determining the estimated cumulative kidney dose.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is an accurate prediction of kidney dose in 177Lu-PSMA RLT in prostate cancer patients feasible with reduced SPECT/CT measurements for dosimetry?
PERTINENT FINDINGS: A simplification of intratherapeutic imaging protocols by performing dosimetry only at the first or every other therapy cycle is feasible.
IMPLICATIONS FOR PATIENT CARE: A reduction of dosimetry measurements improves patient comfort and frees scanner and staff capacities, but an individual kidney dosimetry is essential for accurate dose estimation.
Footnotes
Published online June 04, 2021.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication March 5, 2021.
- Revision received May 5, 2022.