Abstract
We proposed that metabolic remodeling in the form of increased uptake of the myocardial glucose analog 18F-FDG precedes and triggers the onset of severe contractile dysfunction in pressure-overload left ventricular hypertrophy in vivo. To test this hypothesis, we used a mouse model of transverse aortic constriction (TAC) together with PET and assessed serial changes in cardiac metabolism and function over 7 d. Methods: Scans of 16 C57BL/6 male mice were obtained using a small-animal PET device under sevoflurane anesthesia. A 10-min transmission scan was followed by a 60-min dynamic 18F-FDG PET scan with cardiac and respiratory gating. Blood glucose levels were measured before and after the emission scan. TAC and sham surgeries were performed after baseline imaging. Osmotic mini pumps containing either propranolol (5 mg/kg/d) or vehicle alone were implanted subcutaneously at the end of surgery. Subsequent scans were taken at days 1 and 7 after surgery. A compartment model, in which the blood input function with spillover and partial-volume corrections and the metabolic rate constants in a 3-compartment model are simultaneously estimated, was used to determine the net myocardial 18F-FDG influx constant, Ki. The rate of myocardial glucose utilization, rMGU, was also computed. Estimations of the ejection fractions were based on the high-resolution gated PET images. Results: Mice undergoing TAC surgery exhibited an increase in the Ki (580%) and glucose utilization the day after surgery, indicating early adaptive response. On day 7, the ejection fraction had decreased by 24%, indicating a maladaptive response. Average Ki increases were not linearly associated with increases in rMGU. Ki exceeded rMGU by 29% in the TAC mice. TAC mice treated with propranolol attenuated the rate of 18F-FDG uptake, diminished mismatch between Ki and rMGU (9%), and rescued cardiac function. Conclusion: Metabolic maladaptation precedes the onset of severe contractile dysfunction. Both are prevented by treatment with propranolol. The early detection of metabolic remodeling may offer a metabolic target for modulation of hypertrophy.
- 18F-FDG PET
- glucose metabolism in heart
- left ventricular ejection fraction
- left ventricular hypertrophy
Myocardial hypertrophy is considered initially an adaptive response to stress. When stress is sustained, however, hypertrophy becomes maladaptive. Both functional and structural changes are accompanied by changes in energy substrate metabolism. A hallmark finding in myocardial hypertrophy is a shift away from fatty-acid to glucose oxidation (1). On the basis of earlier work ex vivo, we have proposed that this metabolic remodeling precedes as well as triggers left ventricular (LV) structural and functional remodeling in pressure-overload LV hypertrophy (LVH) and induces the fetal gene program (2–4). However, this hypothesis has never been evaluated in vivo. For this purpose, we used the mouse model of LV pressure overload after transverse aortic constriction (TAC) as a clinically relevant model to assess metabolism and function of the heart subjected to pressure overload using 18F-FDG PET imaging.
A compartmental modeling technique was used to analyze images obtained dynamically to compute the rate of myocardial 18F-FDG uptake and use. The quantification of these rates has been limited by the following 2 important factors: first, the limited spatial resolution of the small-animal PET scanners (5,6) leading to partial-volume (PV) effects, and second, the spillover of radioactivity from the blood pool to the myocardium, and vice versa. A major shortcoming of the image-derived blood input function (IDIF) method is that it is susceptible to spillover and PV effects (7).
In this study, a (compartment) model–corrected blood input function (MCBIF) was optimized, wherein the blood input function with spillover and PV corrections and the metabolic rate constants in a 3-compartment model were simultaneously estimated from ordered-subset expectation maximization maximum a posteriori (OSEM-MAP) cardiac and respiration-gated PET images with attenuation correction (8). LV ejection fraction (LVEF) was also measured using high-resolution gated PET images, enabling us to perform a side-by-side comparison of cardiac metabolism and LV function.
MATERIALS AND METHODS
Animal Model
Sixteen adult C57BL/6 male mice (age, 9–10 wk) obtained from Charles River were imaged at baseline using a microPET Focus-F120 scanner (Siemens, Inc.) under sevoflurane anesthesia (9). A subset of these mice (n = 11) was subjected to a TAC surgical procedure to induce pressure-overload hypertrophy (10). Briefly, mice were anesthetized with isoflurane and intubated for ventilation. A thoracotomy was performed, and a 7-0 silk suture was tied around the transverse aorta between the proximal and distal carotid arteries against a 27-gauge needle, after which, the needle was removed. Osmotic mini pumps containing either propranolol (5 mg/kg/d; Sigma Aldrich Inc.) (n = 6) or vehicle alone (n = 5) were implanted subcutaneously at the end of surgery. In addition, 5 sham-operated animals were subjected to the same surgical protocol, without tying off the suture. Before and after surgery, all mice were kept in an environment of 12-h light/12-h dark. Before imaging, animals were (11) overnight with access only to water.
PET scans were obtained between 9 am and 5 pm on anesthetized animals. All experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals (12) and were conducted under protocols approved by the Institutional Animal Care and Use Committee at the University of Virginia.
Small-Animal PET Imaging
PET imaging for measuring myocardial 18F-FDG uptake and glucose utilization was performed using 18F-FDG in the sham mice, TAC mice, and TAC mice treated with propranolol as follows: after the insertion of a tail-vein catheter, electrocardiograph surface electrodes (Blue Sensor; Ambu Inc.) were placed on both forepaws and the left hind paw, and a pneumatic respiratory pillow was placed on the animal’s chest. Dynamic PET scans (60 min) were obtained under 2.5% sevoflurane anesthesia in oxygen (9), where data acquisition was initiated a few seconds before the slow administration of about 29.6 MBq (800 μCi) of 18F-FDG over 30–60 svia the catheter. A Small Animal Monitoring and Gating System (model 1025 L; Small Animal Instruments, Inc.) for PET imaging was used to continuously monitor heart rate, respiration, and core body temperature (rectal probe). Cardiac gate signals were generated at the end-expiration phase of the respiratory cycle to time-stamp the PET scanner data for subsequent retrospective reordering into heart- and respiratory-cycle–based time bins (13). Transmission scans using a 57Co point source were obtained for attenuation correction before 18F-FDG administration. The list-mode data (sorted into 23 time bins [11 × 8, 1 × 12, 2 × 60, 1 × 180, and 8 × 400 s] and 3 gates [hence referred to as gated]) were reconstructed using an OSEM-MAP algorithm (14,15) with attenuation correction. For comparison the data were also reconstructed using a filtered backprojection (FBP) algorithm (ramp filter cut off at the Nyquist frequency) with the same spatial resolution without gating. The images were corrected for radioactive decay, random coincidences, and dead-time losses using the microPET Manager (Siemens Inc.). Regions of interest in the area corresponding to the LV blood pool and the myocardium were drawn in the last frame and the last gate of the dynamic image data, and time–activity curves for the LV blood pool and the myocardium were generated for the whole scan duration of 60 min. Blood samples were also collected at around 43 and 56 min after 18F-FDG administration from the tail vein to validate the MCBIF. Three blood samples before and 3 blood samples after the PET scan were drawn by a tail-vein nick to measure blood glucose levels using a glucometer (Accu-Chek; Roche). The average of these blood glucose readings was used to compute the rate of myocardial glucose utilization (rMGU).
An MCBIF (8,16), based on the simultaneous estimation of the blood input function with spillover and PV corrections and the metabolic rate constants, was optimized in this study. The formalism for MCBIF was used to measure the net myocardial 18F-FDG influx, Ki, and hence glucose utilization, rMGU, in vivo in the sham mice, TAC mice, and TAC mice treated with propranolol.
End-systolic volumes (ESV) and end-diastolic volumes (EDV) were also measured by drawing regions of interest at the end-systolic and end-diastolic phases of the heart cycle over multiple slices covering the whole heart from the apex to the base. The volumes obtained in each phase over multiple slices were summed to obtain net ESV and EDV in microliters (μL). The LVEF was measured by the following equation: (EDV − ESV)/(EDV) × 100%.
MCBIF
On the basis of the 3-compartment 18F-FDG model (Fig. 1), the differential equations for 18F-FDG kinetics can be written as follows (17):
Three-compartment 18F-FDG model. Block diagram indicates rate constants K1–k4, between 3 compartments of 18F-FDG kinetic model. Left compartment is vascular space for 18F-FDG, Ca. Middle compartment is extravascular space for 18F-FDG, Ce. Right compartment is cellular space for phosphorylated FDG-6-phosphate (FDG-6-P), Cm. Second and third compartments add up to form myocardial tissue compartment, CT.
Ideally, when a region of interest is drawn within the cavity of the left ventricle, the tissue time–activity curve would equal the whole-blood time–activity curve Cp. However, because of spillover and PV effects, the model equation for an image-derived time–activity curve from the blood pool can be written in terms of fraction of the tissue concentration in the blood compartment and partial recovery of radioactivity concentration from the blood as:
Similarly, for the myocardium tissue, one can write the model equation as:
The optimization was performed by minimizing the objective function (Eq. 10) in the MATLAB programming environment using the function “fmincon,” which is based on an interior-reflective Newton method. The initial guesses and bounds for the PV averaging coefficients (rm, rb) are determined beforehand by performing phantom experiments (14). We have kept the bounds for all the kinetic parameters (K1–k4) and spillover factors (Sbm, Smb) open between 0 and 1. As for the input function, one side of the bounds is determined by the distribution of the input function (A1, A2, A3 > 0 and L1, L2, L3 < 0) whereas the other side is wide open. Optimization of Equation 10 results in simultaneous estimation of the blood input function, Equation 9, and compartment model parameters K1–k4 and hence Ki along with the spillover and PV coefficients (Smb, rb, Sbm, rm), without the need for any blood sampling. Simultaneous estimation results in the blood input function, Equation 9, to be free of spillover contamination and PV-corrected in a 3-compartment model. The rate of myocardial glucose utilization can then be computed as:
Statistical Analysis
Statistical analysis was performed using SigmaStat 3.0 (SPSS, Inc.). Mean values of Ki, [Glu], rMGU, and LVEF were calculated with SE. Two-way repeated-measures ANOVA and Holm–Sidak post hoc tests were used to compare mean values in the sham mice, TAC mice, and TAC mice treated with propranolol at different time points. A P value of less than 0.05 was considered statistically significant.
RESULTS
Quantitative Accuracy of MCBIF Applied to IDIF from OSEM-MAP Gated Images
Representative IDIFs obtained from OSEM-MAP gated images and from FBP ungated images with attenuation correction are shown in Figure 2A. MCBIF applied to IDIF obtained from OSEM-MAP gated and FBP ungated images are also shown in the figure. The venous blood samples at 43 and 56 min are shown for comparison. MCBIF applied to the IDIF obtained from high-resolution gated images correctly accounted for the washout dynamics of 18F-FDG from the blood, when compared with the late venous blood samples. Figure 2B shows MCBIF estimation during the first 5 min of the scan, indicating improved PV recovery when applied to IDIF obtained from OSEM-MAP gated images as compared with that obtained from ungated FBP images.
(A) MCBIF estimation for whole scan duration. Shown are representative IDIFs obtained from OSEM-MAP cardiac- and respiration-gated images and from FBP ungated images with attenuation correction. MCBIF applied to IDIF from OSEM-MAP gated and FBP ungated images is also shown. Venous blood samples at around 43 and 56 min are shown for comparison. MCBIF applied to IDIF obtained from high-resolution gated images correctly accounts for washout dynamics of 18F-FDG from blood, when compared with late venous blood samples. (B) MCBIF estimation during first 5 min of scan. Scan indicates improved PV recovery when applied to IDIF obtained from OSEM-MAP gated images as compared with that obtained from ungated FBP images.
A residual plot of MCBIF applied to high-resolution gated images, when compared with the 2 late venous blood samples obtained from control mice (n = 6), is shown in Figure 3. The analysis revealed an average difference of 0.017 MBq/cm3. The precision (SD of differences) was 0.13 MBq/cm3. The figure also shows the analysis obtained from FBP ungated images. The plot indicated the average difference and precision obtained from the FBP ungated dataset to be 0.28 MBq/cm3 and 0.33 MBq/cm3, respectively. The residual plot exhibits the quantitative accuracy and repetitive behavior of MCBIF when applied to OSEM-MAP gated images.
Residual plot. MCBIF applied to high-resolution gated images, when compared with 2 late venous blood samples, revealed average difference of 0.017 MBq/cm3 in control mice (n = 6). Precision (SD of differences) was 0.13 MBq/cm3. Figure also shows analysis obtained from FBP ungated images. Plot indicated average difference and precision to be 0.28 and 0.33 MBq/cm3, respectively, obtained from FBP ungated datasets. Residual plot indicates quantitative accuracy and repetitive behavior of MCBIF applied to OSEM-MAP gated images.
Metabolic Remodeling in LVH in Mice in Vivo
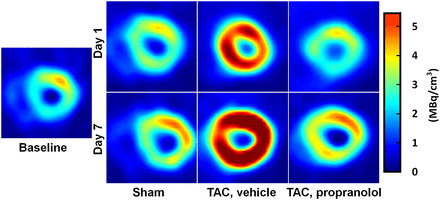
Figure 4 shows representative gated transverse PET images at the last time bin for the sham mice, TAC mice, and TAC mice treated with propranolol at baseline, day 1, and day 7 after surgery. All scans are from the same animals. The images indicate an enlargement in the LV cavity over a period of 7 d in the TAC mice. The images also show an increase in 18F-FDG uptake in the TAC mice starting at day 1 indicative of metabolic adaptation in pressure-overload LVH.
Gated transverse PET images in vivo. End-diastolic transverse PET images at last time bin for sham mice, TAC mice, and TAC mice treated with propranolol at baseline, day 1, and day 7 after surgery are shown. All scans are from same animals. Images indicate enlargement in LV cavity in TAC mice over 7 d. Images also show increase in 18F-FDG uptake in TAC mice starting at day 1, indicative of metabolic adaptation in pressure-overload LVH.
The measured rate of myocardial 18F-FDG uptake, Ki (mL/g/min), for the mice in the 3 categories is shown in Figure 5A over a period of 7 d. The plot shows increased myocardial 18F-FDG uptake at day 1 after TAC surgery by 580%, indicating an adaptive response with preserved LVEF (Fig. 5B). Ki increased further at day 7, with a drop in function by 24%. The blood glucose levels and rMGU are also shown in Figures 6A and 6B for the 3 categories over a period of 7 d. We observed a drop in blood glucose levels at day 1 for the sham mice, TAC mice, and TAC mice treated with propranolol. Mice treated with propranolol showed an elevation in blood glucose levels at day 7, significantly different from day 1 and comparable to the sham group or mice at baseline. The TAC mice exhibited an insignificant increase in blood glucose levels between days 1 and 7 (Fig. 6A). The rMGU (Fig. 6B) did not increase linearly with Ki. Rather, rMGU increased at a much slower rate than Ki, thereby resulting in a mismatch of 29% between Ki and rMGU in the TAC mice (Fig. 7). On the other hand, early treatment with propranolol (25) targeting metabolic alterations served to attenuate the rate of myocardial 18F-FDG uptake (Fig. 5A) reduce mismatch between Ki and rMGU to 9%, and rescue cardiac function (Fig. 5B). The metabolic changes and cardiac function in the TAC mice treated with propranolol were comparable to those in the sham group. The in vivo imaging results suggest that early metabolic remodeling may precede and trigger the onset of severe contractile dysfunction in LVH in mice.
(A) Measured rates of myocardial 18F-FDG uptake in vivo. Ki (mL/g/min) for sham mice (n = 5), TAC mice, vehicle (n = 5) mice, and TAC mice treated with propranolol (n = 6) are shown. Myocardial 18F-FDG uptake at day 1 after TAC surgery is increased by 580%, indicating adaptive response to stress. Ki increased further on day 7 in TAC mice, whereas TAC mice treated with propranolol exhibited attenuated rate of myocardial 18F-FDG uptake over 7-d period. Metabolic changes in TAC mice treated with propranolol were comparable to sham group. All values are mean ± SEM. (B) Measured LVEF from dynamic gated PET images in vivo. Plot indicates insignificant drop in function in TAC mice on day 1. On day 7, function dropped by 24% in TAC mice whereas there is insignificant drop in function in TAC mice treated with propranolol. Sham group indicates no significant change in function over 7-d period. All values are mean ± SEM. *#P < 0.05 vs. baseline, TAC treated with propranolol, and sham groups.
(A) Blood glucose levels. Plot shows average blood glucose levels measured at each time point for sham mice, TAC mice, and TAC mice treated with propranolol. All values are mean ± SEM. *P < 0.05 vs. BSL and day 1. #P < 0.05 vs. TAC at day 7. (B) rMGU was measured at each time point for sham mice, TAC mice, and TAC mice treated with propranolol. All values are mean ± SEM. *P < 0.05 vs BSL, day 1, TAC treated with propranolol, and sham groups. #P < 0.05 vs baseline, TAC treated with propranolol, and sham groups.
Comparison between Ki and rMGU measured in vivo at day 7. rMGU did not increase linearly with Ki. rMGU increased at much slower rate than Ki, resulting in mismatch of 29% between Ki and rMGU in TAC mice. Treatment with propranolol reduced mismatch to 9% and rescued cardiac function.
DISCUSSION
In the normal heart, fatty acid oxidation is the predominant source (60%–90%) of adenosine triphosphate, with glucose oxidation and lactate contributing the rest (26). Stress on the myocardium, such as biomechanical stress from pressure overload, changes normal myocardial substrate metabolism. In the case of pressure-overload–induced hypertrophy, the prevailing notion is that alterations in energy substrate metabolism adversely affect the response to myocardial ischemic stresses and contribute to contractile dysfunction. Specifically, in the hypertrophic heart there is a shift away from fatty acid oxidation toward glucose metabolism (1). At least initially, this metabolic remodeling may be considered as adaptive, most likely because glucose oxidation yields more adenosine triphosphate per atom of oxygen (2). With time, however, the adaptive response becomes maladaptive because this shift in metabolism does not increase adenosine triphosphate production commensurate with the increased demand for energy by the heart.
Recently, we have proposed a requirement for hexose 6-phosphate in mammalian-target-of-rapamycin signaling and cardiac growth (27). However, in the setting of insulin resistance, an oversupply of substrate (glucose) may “starve the heart in the midst of plenty” (28). The hypothesis, however, has never been tested serially in vivo because of the limited availability of technologies for studying complex metabolic parameters and LV function. It is tempting to speculate that strategies to improve myocardial substrate use and to improve metabolic flexibility (3,29) in the hypertrophic heart may improve contractile performance and prevent the onset of heart failure.
During acute pressure overload and inotropic stimulation (30), glucose oxidation is increased, but it is not yet completely clear how glucose uptake is related to LV function during pressure-overload LVH. Studies using SPECT imaging have demonstrated that the metabolic switch can be measured in both small animals and human beings (31,32). However, a serial study on the temporal relationship between glucose uptake and cardiac function in vivo in mice using PET has not been done. We demonstrated that MCBIF applied to an IDIF obtained from high-resolution gated images with less severe spillover and PV effects is quantitative and also repetitive as compared with that obtained from low-resolution ungated images (16). The results of our present study support the hypothesis that an increase in glucose metabolism in the form of increased 18F-FDG uptake precedes and triggers the onset of severe dysfunction of the heart in LVH. This finding is important because early detection of metabolic remodeling may lead to early intervention targeting metabolic changes to prevent the onset of severe contractile dysfunction and improve patient outcomes clinically. We also observed that early treatment with propranolol served to attenuate myocardial glucose uptake and rescue cardiac function in vivo. Thus, metabolic imaging with 18F-FDG PET may provide an early indication of a beneficial effect of a therapy, possibly presenting a platform for aggressive treatment strategies to improve outcomes clinically.
Dietary conditions affect 18F-FDG PET imaging studies (33). Some clinical studies use glucose load conditions without fasting to obtain higher-quality images. On the other hand, fasting can produce similar-quality images. Because we are interested in 18F-FDG dynamics, a reasonable contrast between the blood pool and the myocardial tissue enables us to draw regions of interest and determine rate of myocardial 18F-FDG influx with spillover and PV corrections. Moreover, performing glucose clamping in addition to the TAC surgery in mice is challenging, thus we resort to overnight fasting for our 18F-FDG PET scans to achieve uniform metabolic conditions to obtain Ki and rMGU.
The LC is an important parameter in the determination of the rMGU in a dynamic PET scan. This value may vary, depending on plasma substrate and hormonal conditions. Many authors have used a constant value of LC to compute myocardial glucose utilization in humans with type 2 diabetes (34). Botker et al. (35) proposed that LC is a function of rates of 18F-FDG transport and phosphorylation and applied the method to assess myocardial glucose utilization in humans with ischemic cardiomyopathy (36). In a study by Herrero et al. (37), a constant value of LC was used for assessing myocardial glucose utilization for 18F-FDG PET studies in dogs. However, they also observed that the correlation between Fick-derived and PET-derived rMGU has a lower correlation with the variable LC. The correlation coefficient does not differ after correcting for an LC of 0.67 for 18F-FDG PET studies. In addition, they noted that using the variable LC leads to a significant underestimation of rMGU in large animals. In the current work, we used an LC of 1 because we did not measure this constant and there is no consensus on how to deal with this issue. The concerns expressed earlier by Botker et al. (35) do not apply because here the metabolic conditions were the same for all animals and in steady state. Nevertheless, further studies need to be done to characterize LC in small-animal PET imaging of the heart.
Our study was not without limitations. First, 18F-FDG can be considered only as a surrogate marker for glucose metabolism. Although 18F-FDG indirectly assesses glucose transport and phosphorylation, the tracer analog is subjected to changes in the LC (19). Second, although clinically relevant, the acute nature of the model might have been a limitation in evaluating the hypothesis of metabolic remodeling that precedes and triggers cardiac dysfunction in LVH. The slowly progressive Dahl salt-sensitive hypertensive rat model may be more relevant to evaluate metabolic and functional changes in the heart, in vivo, but the costs for a study such as the present one would be prohibitive (38). Using the 1-kidney, 1-clip rabbit model of hypertension, we have already shown by invasive measurements that metabolic remodeling of the heart precedes hypertrophy (2). Third, we did not consider any possible differences in plasma substrate or hormone levels, although all scans were obtained under the same conditions and any errors would be systematic errors.
CONCLUSION
Noninvasive serial imaging of the heart subjected to pressure-overload hypertrophy using PET supports the hypothesis that changes in the glucose analog 18F-FDG metabolism precede and trigger the onset of severe cardiac dysfunction in LVH. We found that enhanced uptake of 18F-FDG by the myocardium in mice subjected to pressure-overload hypertrophy precedes and possibly triggers the onset of contractile dysfunction. Early intervention with a standard-of-care treatment prevented maladaptive response and rescued cardiac function. We speculate that early detection of metabolic remodeling may offer a metabolic target for modulation of hypertrophy.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by grants HL 102627 and HL 61483 from the U.S. Public Health Service. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Barbara Thornhill for performing the animal surgeries and Gina Wimer for the tail-vein injections during the course of the study.
Footnotes
Published online Feb. 20, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication May 2, 2012.
- Accepted for publication November 12, 2012.