Visual Abstract
Abstract
Encouraging results from targeted α-therapy have received significant attention from academia and industry. However, the limited availability of suitable radionuclides has hampered widespread translation and application. In the present review, we discuss the most promising candidates for clinical application and the state of the art of their production and supply. In this review, along with 2 forthcoming reviews on chelation and clinical application of α-emitting radionuclides, The Journal of Nuclear Medicine will provide a comprehensive assessment of the field.
NOTEWORTHY
TAT shows significant potential in the clinic for cancer treatment.
One of the main challenges limiting the wide application of TAT is the production and supply of suitable TAT radionuclides.
For most TAT radionuclides, current demand significantly exceeds the available supply, but international efforts for increased production are under way.
Targeted radionuclide therapy has seen important clinical breakthroughs, notably originating from the successful clinical translation of prostate-specific membrane antigen–targeted and somatostatin receptor–targeted therapy with β− emitters (notably 177Lu) (1,2). α-emitting radionuclides have also been applied successfully in research and the clinic. Although not a bioconjugate, Xofigo (223RaCl2; Bayer) received clinical approval, representing an important milestone in the translation and application of α-emitter–based radiopharmaceuticals (3).
Improved access to a portfolio of selective α-emitting bioconjugates and radiopharmaceuticals is an important requirement for preclinical evaluations, clinical trials, and translation (4,5). Targeted α-therapy (TAT) combines α-emitting radionuclides with selective delivery systems (e.g., peptides or antibodies). Because of their high linear-energy transfer and high energy (several megaelectronvolts), TAT radiopharmaceuticals deliver therapeutic power within a range of a few cell diameters. This power generates maximal damage to targeted cells while minimizing off-target effects on healthy tissues (6).
Significant efforts are required to optimize the formulation of stable radiopharmaceuticals, determine microdosimetry, and advance clinical studies. However, the major bottleneck for conducting translational research with α-emitters is their limited availability. The high atomic number (Z) of TAT radionuclides, resulting in complex production and lengthy irradiation using powerful reactors or cyclotrons, creates this problem. Alternatively, irradiation of highly radioactive targets at specialized facilities or generation from uncommon isotopes may be required. Therefore, the demand for α-emitters often significantly exceeds availability and supply.
Several comprehensive reviews about various aspects of TAT, including radiochemical considerations (7), and preclinical and clinical applications have been published (8,9). In the current review, we asked a group of experts to highlight research challenges and opportunities for the rapidly evolving field of TAT. We describe the state of the art in production and supply of the most potent clinically relevant α-emitters. We also highlight discrepancies between demand and availability.
Member states have asked the International Atomic Energy Agency to assist with capacity building and technology transfer for the development, production, and quality control of new generations of therapeutic radiopharmaceuticals, including α-emitters. During technical meetings at the International Atomic Energy Agency in 2013 (10), 2018 (11), and 2019, the demand, production routes, radiopharmaceutical aspects, and supply of 225Ac were extensively discussed. The International Atomic Energy Agency will provide guidelines to the member states for production, quality control, preclinical tests, and waste management of α-radiopharmaceuticals.
In the following section, we discuss production and supply aspects of candidates that are currently in clinical practice (227Th/223Ra, 225Ac, 211At, and 212Pb/212Bi) and several promising candidates that are in preclinical evaluation (230U/226Th and 149Tb). All nuclear decay data are taken from the NuDat library, version 2.8 (https://www.nndc.bnl.gov/nudat2/).
CLINICALLY RELEVANT α-EMITTERS
227Th/223Ra
Starting from 227Ac (half-life [t½], 21.77 y), 2 nuclides for TAT applications can be extracted: 227Th (t½, 18.7 d) and 223Ra (t½, 11.43 d) (Fig. 1). Despite their chemical differences, they are grouped because of their common starting material, similarly to 225Ac and 213Bi or 224Ra and 212Pb. Thus, before exploring the current use of 227Th and 223Ra, the production of 227Ac needs to be described.
Decay scheme of 227Ac.
227Ac is produced primarily via neutron irradiation of a 226Ra target in a nuclear reactor (12). Several limitations relevant to 226Ra (t½, 1600 y) as a target material need to be considered: the target is highly radioactive, with the 222Rn (t½, 3.82 d) daughter as a radioactive gas; further, limited quantities of 226Ra are currently available. For an efficient process, a high flux of thermal neutrons with minimum contribution of fast neutrons is preferable, as 226Ra does have a non-negligible fission cross-section for neutrons with energy above 1 MeV (13). Because of the increased focus of medical authorities on production quality, a specification of the target material may be required to ensure the quality of the 227Ac product.
After 226Ra target irradiation, purification of 227Ac from the target is the next step. This is accomplished by separation using liquid chromatography techniques, similar to the procedure for separation of 229Th from 225Ac and 225Ra (14). The separation process needs to remove all radium and all thorium, as both 228Th and 229Th will be present as by-products after irradiation, along with the remaining 226Ra. After 227Ac purification, characterization of the actinium is recommended, to have as much data on the starting material as possible. The 227Ac is typically kept in a dilute nitric solution but may be dried down to actinium nitrate if the material is to be shipped, because shipment of dry material is easier than shipment of a solution, based on the current International Air Transport Association regulations (15).
Alternative approaches include the recovery of 227Ac from legacy actinium-beryllium neutron sources (16) and the accelerator-based production of 225Ac using 232Th as a target generating small quantities of 227Ac as a by-product (17). There was virtually no production of 227Ac between the 1970s and the past decade. Thus, the availability of 227Th and 223Ra was very limited.
227Th is harvested from a generator containing 227Ac. Using separation columns, it is possible to separate thorium from actinium and radium, thus removing both the mother and the daughter nuclides. The purified thorium may be used on-site for immediate labeling or shipping, as thorium chloride, to the labeling site. If shipment or labeling is delayed, the purification step for removal of radium may be repeated to minimize the dose contribution from daughters.
223Ra is also harvested from a generator containing 227Ac. Using separation columns, radium can be separated from actinium and thorium, thus removing both mother nuclides. The purified radium is typically used on-site for drug formulation immediately or shipped as dry radium chloride.
Both 223Ra and 227Th are currently commercially available from Oak Ridge National Laboratory (ORNL) through the U.S. Isotope Distribution Office, and Pacific Northwest National Laboratory, Rosatom, and Bayer have access to 223Ra and 227Th.
Radium was considered a good candidate for TAT, but over the last few decades, no suitable chelator has been found. However, 223Ra in its ionic form is clinically used as Xofigo in the treatment of bone metastatic prostate cancer (18). This does not involve a chelator or a target-seeking moiety. Xofigo thus represents a special form of TAT pharmaceutical. The use and handling of Xofigo, which is currently approved in 53 countries, form the basis for any subsequent TAT pharmaceuticals, including those produced in the European Union, the United States, and Japan.
Because of their short half-lives, all daughters are in radioactive equilibrium with 223Ra at the time of injection. The shelf-life of a radiopharmaceutical will be governed by several parameters, including the activity of the mother nuclide, ingrowth of radioactive daughters, and degradation of the pharmaceutical due to radiolysis of one or several components. In the case of Xofigo, the ingrowth of daughters is not a limiting factor, as the daughters are fully ingrown after some hours. Radiolysis of the pharmaceutical is a concern, particularly radiolysis of the citrate buffer. In addition, the lower specific radioactivity after several half-lives is also a concern. To determine a proper shelf-life, these aspects must be considered and studies must be conducted. In the case of Xofigo, a shelf-life of 28 d was therefore adopted. This shelf-life is unusually long for a radiopharmaceutical, partly because of the t½ of 223Ra and partly because of the low impact of radiolysis, and allows for global distribution independent of production site location. For research applications, the availability of 227Ac, 227Th, and 223Ra is currently sufficient, but the overall supply for clinical or commercial use is less certain. No published data on the production capacity for the different suppliers are available.
225Ac/213Bi
225Ac is one of the most promising TAT radionuclides, with a t½ of 9.92 d and a net emission of 4 α-particles in the decay chain. It can be used for TAT radiopharmaceuticals or as a source of 213Bi (t½, 45.61 min), which also can be applied in TAT (19).
There are several production routes for 225Ac (20). The two most important are separation from the natural decay of 229Th obtained from waste stockpiles containing 233U, and irradiation of 232Th with high-energy protons (>70 MeV) via reaction 232Th(p,x)225Ac.
In addition, irradiation of 226Ra with lower-energy protons (<25 MeV) via the reaction 226Ra(p,2n)225Ac holds great promise for large quantities because of the high (710 mbarn) cross-section peak at 16.8 MeV (21). This reaction could be performed on many of the low-energy cyclotrons already in use for medical isotope production. However, irradiation of a highly radioactive target on these medical cyclotrons, and limited radium quantities, have rendered use of this approach infrequent. Another promising route is photonuclear production via 226Ra(γ,n)225Ra→225Ac, which can provide the clinically relevant supply of 225Ac (22).
Production of 225Ac from 229Th Decay
225Ac is most frequently produced from 229Th generators. 229Th is the grandparent isotope of 225Ac in the decay series of 233U (Fig. 2) and has a t½ of 7,932 y (NuDat), later corrected to 7,917 y (23). As such, 229Th serves as an ideal radioisotope generator for a virtually perpetual supply of 225Ac. The primordial neptunium decay chain to which 229Th belongs is now extinct; therefore, 229Th that is suitable for use via separation technology is of limited availability. The most common source of 229Th is the decay of anthropogenic 233U. Because of safeguarding and nonproliferation efforts surrounding 233U, access to large quantities is limited and approximately only 12.9 GBq (350 mCi) of 229Th have been converted into functioning 225Ac generators to date; this restriction has limited the global annual production of 225Ac to approximately 63 GBq (1.7 Ci) (20).
Decay scheme of 233U.
By allowing the 225Ra (t½, 14.9 d) and 225Ac (t½, 9.92 d) progeny of 229Th to approach secular equilibrium (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org) over typically 30–90 d, the generator can be eluted to separate the shorter-lived daughters. After initial milking of the 229Th generator, 225Ra can be stored for further use as a parent–daughter generator of a reduced but still relevant quantity of 225Ac. The frequency of 229Th and 225Ra generator elution is often determined with considerations for batch size requirements, operational cost, and generator size. Generators with larger amounts of 229Th can produce suitable 225Ac batch sizes with higher frequency. After elution, selective isolation and careful quality control are performed to prepare 225Ac suitable for incorporation into radiopharmaceuticals.
There are multiple 229Th generators in operation, capable of producing 225Ac in quantities relevant for preclinical and limited clinical use. The Directorate for Nuclear Safety and Security of the Joint Research Centre in Karlsruhe, Germany (Supplemental Fig. 2), possesses about 215 mg of 229Th (24), and the Leypunsky Institute for Physics and Power Engineer in the Russian Federation (Supplemental Fig. 3) (25) and ORNL in the United States (26) each possesses 700 mg of 229Th. An additional source recently became available at Canadian Nuclear Laboratories (Supplemental Fig. 4) (27), with 50 mg of 229Th.
All generators rely on an anion exchange mechanism for separation of 229Th from 225Ra and 225Ac. Cation exchange or extraction chromatography is used for 225Ac separation from 225Ra, whereas additional anion exchange processing provides purification from residual thorium (28). These methods produce 225Ac with suitable attributes for preclinical and clinical applications (7). Molar-specific activity and stable metal content can differ for various 225Ac sources. 233U stockpiles are currently being processed at ORNL through a public–private partnership that is expected to yield about 45,000 mg of 229Th (Supplemental Table 1) (29). This material also contains 228Th in sufficient quantities (30) to require exposure shielding due to the presence of 208Tl, complicating generator development and deployment. Work on the generator for this material is ongoing and will leverage the techniques and methods used in the existing generators (31).
Production of 225Ac via Proton Irradiation of 232Th
225Ac has been produced by the spallation reaction 232Th(p,x)225Ac on thorium targets with proton energies ranging from 100 to 1,400 MeV at beam currents of as high as 250 μA. This method of production is presently under development as part of the U.S. Department of Energy’s (DOE’s) Tri‐Lab Effort, involving Brookhaven National Laboratory and Los Alamos National Laboratory for target irradiations and ORNL for subsequent radiochemical processing and dispensing of irradiated targets. The current focus of the U.S. DOE Tri-Lab Effort is to bring colocated processing facilities online at Brookhaven National Laboratory and Los Alamos National Laboratory in the time frame of 6 mo to 5 y, with the goal of greater than monthly production of curie-scale batches. The U.S. DOE Tri-Lab Effort has established processing under current good-manufacturing-practice conditions, with operations captured under a drug master file (32,33).
In addition, efforts are under way to develop spallation production capabilities in Canada using a diverse set of irradiation capabilities at the Tri-University Meson Facility (TRIUMF) (up to 500-MeV proton beams). Producing 225Ac at higher proton energy results in a higher fraction of 225Ac versus 227Ac (Supplemental Fig. 5). This is currently being pursued with TRIUMF’s 500-MeV cyclotron. About 200-MBq (5.4 mCi) quantities of 225Ac are produced with a proton beam of about 480 MeV on target. In addition, significant amounts of 225Ra are also produced, which can be separated and used as a generator isotope for isotopically pure 225Ac (Supplemental Fig. 5). In recent 25 mA·h irradiations of approximately 8-g targets of 232Th, isolation of the radium fraction provided sufficient 225Ra to yield about 18 MBq (∼0.5 mCi) of 225Ac, with no detectable 227Ac (Supplemental Fig. 6 shows the separation, and Supplemental Fig. 7 shows an example of a γ-spectrum (34,35)).
Furthermore, work is under way at the Institute of Nuclear Research, Russia, and at NorthStar Medical Technologies, where teams are developing spallation production targets and new process technologies (36–38).
In thorium spallation, 227Ac (t½, 21.77 y) is coproduced with yields similar to those for 225Ac, leading to concerns about facility licensing and about the path forward for associated waste streams. Overall, 227Ac activity represents approximately 1%–2% of the overall sample activity and has not been demonstrated to impact labeling efficiency with DOTA, the gold standard for radiolabeling, or to result in toxicity concerns (39). The concern related to 227Ac content can be avoided when producing 213Bi from a generator, which retains all actinium isotopes. This issue can also be addressed by isolating radium isotopes and further extracting isotopically pure 225Ac (40). Additionally, researchers at TRIUMF have demonstrated online generation of isotopically pure beams of 225Ac using a resonant laser ionization method (41), and Conseil Européen pour la Recherche Nucléaire (CERN) has demonstrated separation of pure beams from different thick targets of either 225RaF+ or 225Ac+, including molecular ion formation or resonant laser ionization (11). The supply of 225Ra or 225Ac from CERN-MEDICIS (Medical Isotopes Collected from Isolde) or Institute for Transuranium Elements, Karlsruhe, will become available for researchers through a newly approved coordinated European hub, PRISMAP (Production of High Purity Isotopes by Mass Separation for Medical Application). The European medical isotope program kicked off in 2021 (42).
Currently, most 225Ac is used in the form of 225Ac-labeled radiopharmaceuticals (43), both for preclinical development and for clinical studies mainly focusing on the treatment of prostate cancer, neuroendocrine tumors, and gliomas. Although the application of 213Bi, generated from 225Ac/213Bi generators, has also demonstrated significant clinical benefit (44), the limited availability and high cost of high-activity generators are presently hampering further studies.
211At
211At (Fig. 3) is the α-emitting radionuclide that is perhaps easiest to produce. However, its availability has been limited because there are few accelerators in the world that produce an α-beam with the optimal energy range (28–29 MeV) and beam current (10 µA or higher) to produce adequate quantities for research and clinical applications (5,45,46). Additionally, the relatively short t½ of 211At (7.21 h) causes distribution problems. Here, the supply model is a network, as, for instance, established by the DOE isotope program.
Decay scheme of 211At.
The most common method of production uses the 209Bi(α,2n)211At reaction, in which a metallic bismuth target is bombarded with α-particles (Fig. 4). Inexpensive naturally abundant monoisotopic bismuth (available at 99.999%) can be used directly for target preparation. In general, the bismuth metal is melted onto or is deposited from a vapor onto an aluminum or copper backing. Bismuth metal, although inexpensive, is a poor thermal conductor and has a low melting point (272°C). Thus, effective cooling methods to prevent the target from melting during irradiation are required (47). Thick targets (80 μm) are desired for production and to keep the beam from hitting the target backing, but thinner targets allow for the most efficient cooling. Alternate bismuth target materials with higher melting points, such as Bi2O3, can be used in irradiation, but thus far none have proven to be superior to bismuth metal in the production of 211At.
Direct and indirect production routes of 211At. 211Rn can also be produced by spallation of actinide targets (uranium or thorium) induced by high-energy protons (reaction not shown).
The incident energy of the α-beam in bismuth irradiations is important for optimizing 211At production rates and for minimizing production of an unwanted radionuclide, 210At. Production of 210At (t½, 8.1 h) is problematic as it decays to a long-t½ α-emitter, 210Po (t½, 138.38 d). Although 210Po is found in nature, it has high human toxicity. Generally, a 28-MeV α-beam has been used to preclude 210At production. In an optimization study at 29 MeV, no quantities of 210At were detected (48). At 29 MeV, the production rate of 211At was increased by about 15% over that of a 28-MeV irradiation. Production of 211At can be significantly increased by increasing the accelerator beam current, but unfortunately, the feasible beam current is inherent in the design of the accelerator. However, a higher beam current can be obtained by irradiating an internal target as the beam current is lost during extraction to external beamlines (49).
Isolation of pure 211At from irradiated bismuth targets is also relatively simple compared with other α-emitters, as there are no other radionuclides produced under optimal irradiation conditions. The most common and perhaps simplest method for isolation of 211At is high-temperature (650°C–700°C) dry distillation. However, there can be radiation safety concerns with volatilized 211At. Therefore, alternative wet chemistry isolation methods are being developed (50,51). To simplify the isolation of 211At, methods for automation of dry distillation and wet chemistry approaches are being developed (52).
Current quantities of 211At are inadequate for widespread clinical use. In fact, only Duke University and the University of Washington in the United States, and Copenhagen University Hospital in Denmark, have produced 211At for clinical trials.
The U.S. DOE Office of Isotope Research and Development and Production provides funding to improve the availability of 211At in the United States. It is also creating a university network for 211At production in different regions of the United States for shipment to users through the DOE National Isotope Development Center. Japan has 5 sites producing 211At by α-beam irradiation for use at 13 sites. In support of research, the European Union has recently initiated a cost action (CA19114) that involves networking of 211At production centers among several European countries.
Although production of 211At is currently limited, accelerators with medium-energy α-beams can be added at a significantly lower cost than high-energy accelerators or nuclear reactors required for the production of other α-emitters. Accelerator technology innovations could provide much higher α-beam currents than do current systems. Although new target technology will be required with higher α-beam currents to circumvent target melting, ultimately much larger quantities of 211At could be produced.
An alternative, early-stage, research approach for 211At production involves irradiation of bismuth metal with lithium ions to produce 211Rn (Fig. 4) for a 211Rn/211At generator. Since 211Rn has a t½ of 14.6 h, its decay during transit might provide a more effective distribution of 211At. Radon is classified as a gaseous element and may not be suitable for chemical operations. However, since it has a high affinity for nonpolar organic solvents, it is possible to make a generator by applying the solvent extraction method (53). Such a generator system has been demonstrated in which gaseous 211Rn was isolated and retained in liquid alkane hydrocarbon (dodecane), and 211At generated from the 211Rn source was extracted in an aqueous solution (2N NaOH) (54).
212Pb/212Bi
Both 212Bi (t½, 60.55 min) and 212Pb (t½, 10.64 h) are part of the 232Th (t½, 1.4 × 1010 y) and 232U (t½, 68.9 y) decay chain, with 212Bi being the decay daughter of 212Pb (Fig. 5). 212Pb emits 2 β−-particles and 1 α-particle through its decay to stable 208Pb, whereas 212Bi emits 1 β−-particle and 1 α-particle, which can be used for targeted radionuclide therapy. Either radionuclide is commonly isolated from 228Th sources, which is a decay daughter of both 232Th and 232U.
Decay scheme of 232Th and 232U.
A major drawback to using 212Bi clinically is the emission of a relatively intense and very high energy γ-ray (2.6 MeV of 36% intensity per decay of 212Bi) via its daughter 208Tl. This also creates an obstacle to handling 228Th sources, leading to stability issues due to radiolytic damage of generator systems, and mandates significant shielding for operators (55). These issues have been noted for the newly available 229Th, which contains 228Th in relatively high quantities (as described in the “225Ac/213Bi” section).
Using 212Pb as an in vivo generator, instead of 212Bi directly, in radiopharmaceutical development reduces the amount needed for therapy 10-fold and facilitates radiopharmaceutical production, formulation, and administration given its longer t½. However, the issue of daughter recoil after β-decay and subsequent retention of progeny needs to be taken into consideration (56), similarly to the other α-emitters discussed.
212Pb, and thus 212Bi, are isolated from 228Th or 224Ra generators, both of which are natural decay products of 232Th. 228Th can be obtained through isolation of 228Ra at annual intervals from 232Th or by isolation from anthropologic sources via 232U stockpiles (a portion of which has been transferred by the Department of Defense to AlphaMed, Inc., from stocks at ORNL, or by the double-neutron capture and successive β− decay of 226Ra (57)).
Isolation from natural 228Ra remains difficult given the need to process tons of aged 232Th to obtain useable amounts. Each ton of more than 35-y-old 232Th can yield approximately 3.7 GBq (100 mCi) of 228Ra. The company OranoMed extracts 212Pb from natural thorium salt. Subsequent separation, purification, and concentration of elements decaying from 232Th provide worldwide shipment of 212Pb generators.
228Th can be produced from successive neutron capture and β− decay of 226Ra. In the past, this production was proven to be feasible, but further process development is needed to determine production yields and cost.
Around 555-MBq (15 mCi) 224Ra/212Pb/212Bi generators are available through the U.S. DOE Isotope Program (via ORNL) (58). The current generator is sufficient only for preclinical development (not clinical use), as radiolytic damage limits the scale-up (59). Briefly, 224Ra (t½, 3.63 d) is separated from immobilized 228Th adsorbed onto an organic cation exchange resin (highly cross-linked MP-50, ∼300 μL in volume). A 212Pb and 212Bi mixture is eluted with a few milliliters of 2 M HCl or 0.5 M HI with approximately 70% yield and parent breakthrough of 10−6. It is also possible to elute 212Bi (free from 212Pb) selectively with 0.5 M HCl or 0.15 M HI. The 224Ra/212Pb/212Bi generator has a shelf-life of about 2 wk.
Westrøm et al. (60) prepared a 228Th/224Ra generator based on thorium purchased from Eckert and Ziegler. In this process, 228Th was immobilized on a DIPEX (Eichrom) actinide resin by mixing 228Th in 0.1 M HNO3 with a portion of the actinide resin and, after a few hours, loading onto a column containing a small portion of inactive actinide resin to avoid breakthrough. 224Ra could be eluted regularly from the generator column with 1 M HCl.
McNeil et al. (61) reported the preparation of a novel 228Th/212Pb generator using 228Th produced as a by-product of 232Th spallation with 500-MeV protons at TRIUMF. The bulk thorium (8 g) (coprecipitated with 228Th) was purified via anion exchange resin. 228Th did not absorb to the column and was found in load and wash fractions, which were collected, evaporated to dryness, and redissolved in 1 M HNO3 to produce the generator stock solution. The 212Pb was separated from 228Th by passing the generator stock solution through an 80-mg lead resin (Eichrom).
Research Candidates
There are several hundred α-emitters in the chart of radionuclides; however, most are not suitable for TAT because of their t½ or difficulties with the production and formulation of radiopharmaceuticals. However, with emerging alternative production routes and advancement in chelation systems, several additional candidates are of interest for TAT.
230U/226Th
230U (t½, 20.8 d) can be used for TAT directly or as a generator source of shorter-lived 226Th (t½, 30.57 min). One potential advantage over 225Ac/213Bi is that 230U/226Th has multiple α-decays with very short lived daughters (seconds), which potentially may prevent significant delocalization of daughters after the decay from the targeting site (Fig. 6). One of the main challenges in using 230U in the same way as 225Ac for direct labeling of biomolecules (e.g., antibodies or peptides) is the still relatively undeveloped chelation of uranium for radiopharmaceutical application. In addition, for the 230U/226Th generator, the shorter t½ of 226Th may represent challenges in term of logistic and radiopharmaceutical synthesis when used in the same way as 213Bi. 230U can be produced either directly by proton or deuteron irradiation of 231Pa (t½, 3.276 × 104 y) (62,63) or by decay of 230Pa (t½, 17.4 d), which can be produced by spallation of 232Th (64–66). For the first route, the production rate is 0.25 MBq (6.7 μCi)/μAh for 25 MeV of energy (65), and limiting factors are the handling and availability of the target material. For the second route, the main limitation is that only 7.8% of produced 230Pa decays to 230U, significantly decreasing the final yield. 230Pa can also be produced as a by-product during proton spallation of 232Th and coextracted along with other medical radionuclides (67), such as 225Ac. Radiochemical processing is required for the protactinium, uranium, and thorium, as is already well known and can be achieved by a combination of ion-exchange and solid-phase extraction chromatography. On the basis of the similar required clinical quantities of 225Ac, 230U can be produced (gigabecquerels [tens of millicuries]) via both routes; however, the currently low demand and absence of a suitable chelation system for uranium limit application of this attractive radionuclide.
Decay scheme of 230Pa.
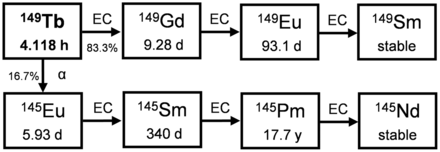
149Tb
149Tb (t½, 4.118 h) is the lightest α-emitter if one excludes those with the extremely short (108Te) or long (146Sm) t½. 149Tb was recognized long ago as an α-emitting radionuclide with potentially interesting properties (Fig. 7), as it was found to be capable of killing single cancer cells in vitro and is part of a terbium theranostic quadruplet covering the different nuclear medicine modalities in therapy and diagnostics (68–70). Cyclotron irradiation of gadolinium targets with high-energy protons (70–200 MeV) and spallation reactions of protons at high energy (>1 GeV) on thick tantalum targets are the most favorable production routes. In all cases, mass separation is required to suppress coproduced terbium radionuclides, with demonstrated efficiencies of 12% at CERN-MEDICIS and of 50% with stable terbium tracers at the LARISSA (Laser Resonance Ionization Spectroscopy for Selective Applications) isotope separator at the University of Mainz (Germany) and for the 149Dy production route followed at ISOLDE (Isotope Mass Separator On-Line). Daily cycles of 500-MBq batches are expected from 2021 onward at CERN-MEDICIS to produce no-carrier-added 149Tb radionuclide batches.
Decay scheme of 149Tb.
The relatively short t½ of 149Tb implies distribution networks mimicking those of many diagnostic radiopharmaceuticals. To support 149Tb distribution, transportation limits have been updated accordingly in 2018 and are no longer a limiting factor for the dispatch of relevant activities for clinical applications (71).
Although these different α-emitters have been made available to researchers under different access modalities, a new consortium has been established, funded by the European Union’s Horizon 2020 research and innovation program. PRISMAP comprises important nuclear reactors, accelerators, and isotope mass separation centers. It aims at providing different radionuclides for medical researchers through a single hub, a single web platform (42). A call for projects, selection by a user panel, and determination of important nuclear data for proper standardization are fully included in the project’s implementation. PRISMAP started on May 1, 2021.
DISCUSSION
The current supply of most TAT radionuclides is insufficient for preclinical and clinical evaluation. Only very few research groups have reliable access to TAT radionuclides, because of either high costs or long wait times. Therefore, the supply of α-emitting radionuclides for TAT is a pressing issue that needs to be addressed urgently. The current review intends to stimulate discussion and provide useful information on the selection and handling of TAT radionuclides for scientists and clinicians who would like to develop TAT programs. Table 1 summarizes the nuclear properties, current availability on a clinical scale, and the potential for a future increase in production.
Nuclear Properties of Discussed TAT Radionuclides and Their Current Production Route*
Several strategies can potentially solve the supply shortage of TAT radionuclides: development of alternative production strategies by exploiting existing infrastructure or developing novel approaches; improvement of targeting and radiochemical separation strategies enabling scaling up of the production of TAT radionuclides; achieving a fieldwide consensus on the appropriate radionuclidic and radiochemical purity for preclinical and clinical applications; development of a broad portfolio of different chelation and delivery systems to mitigate the physical and chemical limitations of α-emitters; and definition of dosimetry and quality standards for clinical applications.
CONCLUSION
The supply and production of α-emitters are of critical importance when selecting the most suitable candidates for preclinical and, in particular, clinical TAT. The availability of α-emitters has slowed the successful development of radiopharmaceuticals for TAT. Nevertheless, several landmark developments, including the success of Xofigo and 225Ac-labeled prostate-specific membrane antigen ligands, demonstrated the great potential of TAT. The strong academic and industry interest further stimulated by these success stories is expected to significantly improve radiopharmaceutical supply soon.
DISCLOSURE
TRIUMF receives funding via a contribution agreement with the National Research Council of Canada. Funding is also acknowledged through the CERN & Society Foundation, the Flanders Research Foundation (FWO), the CERN Knowledge Transfer Fund, and the European Commission (MEDICIS-Promed, H2020 contract 642889). This paper was stimulated by the successful application of the PRISMAP project, the European Union’s Horizon 2020 research and innovation program, under grant 101008571. Marek Pruszyński acknowledges support from the European Union Horizon 2020 research and innovation program under grant 857470 and from the European Regional Development Fund via the Foundation for Polish Science International Research Agenda PLUS program grant MAB PLUS/2018/8. This research and the production of 211At at the University of Washington are supported by the U.S. DOE Isotope Program, managed by the Office of Science for Isotope Research and Development and Production. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENT
We acknowledge Dr. Gokce Engudar (TRIUMF) for designing and producing the graphical abstract.
Footnotes
Published online July 22, 2021.
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 5, 2021.
- Accepted for publication June 29, 2021.