Abstract
Emotional and cognitive abnormalities are common in adult hypothyroidism. Few studies, however, have evaluated cerebral perfusion and metabolism in this disorder. The aims of this study were to compare regional cerebral blood flow (rCBF) between hypothyroid patients and healthy subjects and assess flow during the euthyroid state after treatment. Methods: Ten mildly hypothyroid patients, before and after thyroxine treatment, and 10 healthy controls underwent 99mTc-hexamethylpropyleneamine oxime brain SPECT, MRI, and psychometric testing. SPECT images were analyzed using statistical parametric mapping. Results: Compared with controls, rCBF in patients before treatment was lower in right parietooccipital gyri, cuneus, posterior cingulate, lingual gyrus, fusiform, insula, and pre- and postcentral gyri. Perfusion did not normalize on a return to the euthyroid state. Conclusion: Decreased rCBF in mild hypothyroidism is found in regions mediating attention, motor speed, memory, and visuospatial processing, faculties affected in hypothyroidism. Follow-up studies are needed to determine the longer-term persistence of perfusion abnormalities in this disorder.
Thyroid hormone plays a crucial role in mammalian central nervous system cell division, maturation, and function (1). In the pre- and perinatal periods, thyroid hormone deficiency is associated with severe cognitive impairment and growth retardation (2). Although the effects of thyroid hormone on the adult central nervous system are not so drastic, it is also sensitive to the thyroid hormones.
Mild adult-onset hypothyroidism is a common condition, accompanied by emotional symptoms, including lethargy and dysphoria, and by cognitive decline, including deficits in attention, short-term memory, and visuospatial processing (3–5). Even subclinical hypothyroidism is associated with depressive symptoms and cognitive impairment (6,7). Yet, the effect of thyroid status on cerebral blood flow (CBF) and metabolism has not been widely investigated. Early studies showed a decrease in mean global CBF in adult hypothyroidism, with a return to normal in the euthyroid state (8–10). Using 31P-magnetic resonance spectroscopy, Smith and Ain documented increased oxidative metabolism in the frontal lobes of 10 postthyroidectomy patients after thyroxine treatment (11). A recent MRI study showed an increase in brain volume and decrease in ventricular size in 3 severely hypothyroid patients after thyroxine treatment (12). Marangell et al. (13) used PET to study correlations between thyroid hormones, regional CBF (rCBF), and glucose metabolism in depressed patients with normal or slightly raised thyroid-stimulating hormone levels (TSH). An inverse correlation between TSH and rCBF was reported in the left prefrontal cortex, whereas an inverse correlation between TSH and cerebral glucose metabolism was seen in the right cuneus, but these findings have not been extended to hypothyroid populations. Constant et al. (14) used PET to study CBF and glucose metabolism in 10 patients who had undergone thyroidectomy for thyroid carcinoma during a euthyroid state maintained by thyroxine, and in severe acute hypothyroidism before treatment. In the hypothyroid state, global CBF was lower in all patients, whereas glucose metabolism was lower in 8 of 10 patients. However, it is difficult to determine whether this population is representative of nonmalignant hypothyroid physiology.
The aim of the present study was to use SPECT to investigate rCBF in patients with mild adult-onset hypothyroidism before and after treatment with thyroxine.
MATERIALS AND METHODS
Subjects
Ten patients (all women; mean age, 45.9 ± 15.1 y; range, 21–64 y) with newly diagnosed adult-onset hypothyroidism due to atrophic or Hashimoto’s thyroiditis and 10 healthy volunteers (9 women and 1 man; mean age, 49.7 ± 11.3 y; range, 29–64 y) participated in the study. All patients were mildly symptomatic, with elevated TSH, low T4 levels, and normal T3 levels, as evaluated immediately before initiation of thyroxine treatment. The duration of the reported hypothyroid state before the first SPECT scan ranged from a few weeks (in 6 patients) to 7 mo. Normal TSH was verified in all control subjects. Exclusion criteria for patients and control subjects included cardiovascular, neurologic, or psychiatric (DSM-IV axis I) disorders, past head trauma, abnormal brain MRI findings, a Haschinsky Ischemia Index score > 2, or treatment with somatic medication that might affect the SPECT evaluation. The study was approved by the Hadassah University Hospital Ethics Committee. Informed consent was obtained.
Study Design
Hypothyroid patients were evaluated by 2 SPECT studies: the first when the patient was hypothyroid, and the second when euthyroid. The first SPECT scan was acquired within 7.9 ± 6.5 d (range, 0–21 d) of a TSH assay, and the second scan was acquired within 21.2 ± 11.9 d (range, 4–43 d) after a second TSH assay documenting the euthyroid state. The interval between scans was 109 ± 44 d (range, 63–215 d), reflecting the time required to achieve a euthyroid state in these subjects. Control subjects underwent a single SPECT study.
To document mood and exclude gross cognitive dysfunction, the Hamilton Rating Scale for Depression (HAM-D) (15) and the Mini-Mental State Examination (MMSE) (16) were administered at baseline for both patients and healthy volunteers and were repeated for patients at the time of the second scan.
SPECT Acquisition and Image Processing
The subjects were injected with 740 MBq of 99mTc-hexamethylpropyleneamine oxime in a quiet, dimly lit room, while supine with open eyes and unplugged ears. The SPECT acquisition began 30 min later, with the head immobilized on a headrest and secured with hook-and-loop straps.
Images were obtained with a dual-head, rotating γ-camera equipped with a fanbeam collimator (HELIX; Elscint). Data were collected on a 128 × 128 matrix in 60 projections, at 25 s per projection. Processing included normalization, transaxial reconstruction using filtered backprojection, and theoretic attenuation correction. Reconstruction was performed with a Hanning filter on a 64 × 64 matrix, using a slice thickness of 1 pixel (4.7 mm). The resolution of the system is 11 mm full width at half maximum.
Data Analysis
Scan data were analyzed using statistical parametric mapping (SPM99; Wellcome Department of Cognitive Neurology) on a MATLAB platform (The MathWorks, Inc.). Images were realigned to the Montreal Neurologic Institute brain atlas, resampled to pixel size 2 × 2 × 2 mm, and smoothed with an isotropic gaussian kernel with a 10-mm full width at half maximum. Each image was normalized to mean total activity within the brain. Pixels below 80% of the global mean were removed. For all SPM analyses, the initial voxel threshold was set at P < 0.01 and minimum cluster extent was 30 voxels; only clusters with corrected cluster P < 0.05 were reported as significant.
Unpaired t tests were used for group comparisons between hypothyroid and control subjects, and a paired t test was used to compare pre- and posttreatment scans within the hypothyroid group. Correlation analysis between rCBF in the hypothyroid subjects before treatment and TSH, HAM-D, and MMSE was performed for each variable while controlling for possible effects of the other two. We also tested for a correlation between pre/posttreatment scan differences and change in TSH or treatment duration.
RESULTS
In the hypothyroid patients, TSH decreased significantly after treatment (from a mean of 15.1 ± 2.9 mU/L to a mean of 1.7 ± 1.2 mU/L, P < 0.0001). In the control subjects, the mean TSH was 1.7 ± 0.8 mU/L and the range was 0.5–2.9 mU/L. In hypothyroid patients at baseline, free T4 (available for 5 subjects) was 9.3 ± 1.6 pmol/L and the range was 7.4–11.5 pmol/L; total T3 (available for 4 subjects) was 2.4 ± 1.0 nmol/L and the range was 1.7–3.8 nmol/L, in keeping with mild hypothyroidism, in which T3 levels are within the normal range. At baseline, the hypothyroid patients showed moderate dysphoria (HAM-D, 10 ± 5.6: normal score is <7; score for depression is >18–20), with a nonsignificant decrease to 5.2 ± 5.8 (P = 0.4) at the euthyroid state. The cognitive function of the hypothyroid patients was intact both before and after treatment, with a nonsignificant increase in MMSE (from a mean of 28.3 ± 2.0 to a mean of 29.4 ± 1.6, P = 0.2). No correlation was found between TSH and HAM-D scores (R = 0.28, P = 0.44), between HAM-D and MMSE scores (R = −0.07, P = 0.84), or between TSH and MMSE scores (R = −0.46, P = 0.19) in hypothyroid patients before treatment.
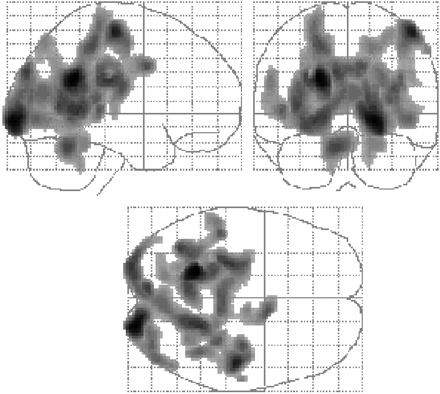
SPM analysis showed a significantly lower rCBF in the mildly hypothyroid patients before treatment, mostly in posterior parts of the brain, including parts of the parietooccipital cortex and temporal lobes (Table 1; Fig. 1). Compared with control subjects, hypothyroid patients after treatment again revealed hypoperfusion, with a single large region essentially encompassing the 4 separate smaller regions seen before treatment (Table 2; Fig. 2). No regions of significantly higher rCBF were observed in the hypothyroid patients either before or after treatment. Comparison of patient scans before and after treatment yielded no significant differences. Thus, despite a return to the euthyroid state, regions of hypoperfusion remained in the hypothyroid patients after treatment.
Regions of significantly lower rCBF in mildly hypothyroid patients before treatment than in control subjects. Some clusters are large and extend into many brain regions.
Regions of significantly lower rCBF in mildly hypothyroid patients after treatment than in control subjects.
Regions in Which Hypothyroid Patients at Baseline, Before Treatment, Show Lower rCBF Than Do Control Subjects
Regions in Which Hypothyroid Patients, After Treatment, Show Lower rCBF Than Do Control Subjects
Comparisons between small patient groups may be unduly dominated by individual scans. To avoid this problem, all SPM comparisons were repeated, omitting one subject at a time. Results were not significantly affected by omission of individual hypothyroid or control subjects, confirming that our results reflect phenomena seen across groups.
In both hypothyroid patients (pretreatment) and control subjects, no regions of significant positive or negative correlation were observed between rCBF and TSH, HAM-D, or MMSE or between pre/posttreatment scan difference and change in TSH or treatment duration.
DISCUSSION
In this study, we found a significant reduction in relative rCBF in patients with mild adult-onset hypothyroidism. This reduction was maintained after resumption of the euthyroid state after thyroxine treatment. No correlation was found between severity of hypothyroidism, measured by TSH before treatment, and rCBF.
These findings raise questions about possible mechanisms by which thyroid hormones might affect rCBF. Decreased global CBF in hypothyroidism was interpreted as a consequence of increased vascular resistance (8,9). The mechanism by which low levels of thyroid hormones reduce regional cerebral perfusion is unclear. Contrary to expectation, the amygdala and hippocampi, regions rich in nuclear thyroid hormone receptors, did not exhibit reduced rCBF, suggesting that thyroid regulation of rCBF is not mediated by these receptors but rather by extranuclear processes. Such processes, associated with regulation of vasodilation in hypothyroidism, would not explain the regional, rather than global, perfusion deficits. Likewise, generalized systemic phenomena associated with hypothyroidism, such as increased vascular resistance and decreased cardiac output, would cause global perfusion reductions that would not be picked up by our relative semiquantitative analysis and would not explain the regional deficits in this study.
The rCBF deficits in this study also have implications for the cognitive and emotional deficits associated with hypothyroidism. rCBF reductions in the right primary motor cortex may be related to motor retardation and psychomotor slowness typical of hypothyroidism. Perfusion deficits in the posterior cingulate and fusiform gyri, insula, and right parietooccipital cortex may affect attention and working memory, written word recognition, retrieval of episodic memory, perception and imagery of visuospatial input, and priming processes (17), often compromised in hypothyroidism (4). Although not revealing significant cognitive impairment in our cohort, MMSE is a rather crude measure of cognitive function, and previous studies examining patients with hypothyroidism of similar or lesser severity reported cognitive deficits that were not always completely restored after treatment (7,18,19). Use of a more sensitive instrument might shed further light on the association between hypothyroidism-related regional perfusion deficits and cognitive function.
Normalization of decreased global CBF on a return to the euthyroid state has been previously reported (8,10,14). However, all previous studies examined pre/posttreatment within-group measures and did not compare patients with healthy volunteers (14). Thus, it is not known whether cerebral perfusion in either the hypothyroid or the euthyroid state is similar to that of healthy volunteers. Our findings suggest this may not be so. Unlike the hypothyroidism-associated global CBF decreases, regional blood flow deficits in our cohort did not change after resumption of the euthyroid state. In addition, the patient population in other studies consisted of postthyroidectomy patients, and the effect of severe, abrupt hypothyroidism on rCBF may be different from that of mild hypothyroidism. The persistence of relative rCBF decreases may reflect continuing subtle neurocognitive deficits (4,5,7,18,19) not captured in our study. Alternatively, regional perfusion deficits may require a longer time to normalize or may represent an abnormal trait pattern typical of hypothyroidism.
CONCLUSION
Our findings suggest that widespread reductions in rCBF occur in mild hypothyroidism and persist after a return to the euthyroid state. Although the effects of hypothyroidism on rCBF could shed light on the mechanism of action of thyroid hormones on brain metabolism, and thus on the symptoms of hypothyroidism, this preliminary short-term study was performed on a small number of mildly hypothyroid patients, and the significance of the findings remains unclear. Long-term, prospective studies on larger cohorts are necessary to replicate our findings, to investigate the effects of prolonged thyroxine treatment, and to study patients with subclinical and more severe hypothyroidism.
Acknowledgments
This study was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award to one of the authors.
Footnotes
Received Feb. 15, 2004; revision accepted Apr. 23, 2004.
For correspondence or reprints contact: Yodphat Krausz, MD, Department of Nuclear Medicine, Hadassah-Hebrew University Medical Center, P.O. Box 12000, Jerusalem 91120, Israel.
E-mail: yodphat{at}hadassah.org.il