Abstract
Cancer therapy has advanced with molecularly targeted approaches and immunotherapy, yet chemotherapy remains essential for many aggressive cancers, including breast, lung, ovarian, pancreatic, bladder, sarcoma, and lymphomas. A major challenge is chemoresistance, in which cancer cells evade chemotherapy’s cytotoxic effects. Overexpression of adenosine triphosphate–binding cassette transporters, especially P-glycoprotein, significantly contributes to this resistance. Thus, imaging biomarkers are urgently needed to detect P-glycoprotein overexpression in vivo, identify resistant cancer cell clones, and map their distribution and heterogeneity within tumors. This article reviews the applications of SPECT, PET, and optical imaging in addressing chemoresistance. It emphasizes the potential of these modalities to enhance cancer treatment by enabling early identification of resistant clones and improving therapeutic strategies. The article outlines key steps required for the integration of molecular imaging into clinical practice, aiming to overcome chemoresistance and optimize patient outcomes.
In 2024, the annual report released by the American Cancer Society, which estimates new cancer cases and cancer-related deaths, provided 2 interesting and seemingly contradictory data points (1). The first, reassuring, is that cancer mortality has been steadily declining since 2021, mainly because of the reduction in smoking habits and the implementation of new therapeutic strategies, such as immunotherapy and molecularly targeted therapy. The second alarming data point, however, is the increased incidence of the most common cancers in the population, such as breast, pancreas, prostate, colorectal, and uterine corpus cancers. Although these findings push for the development of innovative therapeutic strategies, they also highlight the need to further investigate the mechanisms underlying resistance to the most common and, in some cases, first-line cancer treatment: chemotherapy with cytotoxic drugs (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org).
Nuclear medicine, through SPECT or PET, offers a unique opportunity to investigate physiologic and pathologic processes at the molecular level. The development of radiopharmaceuticals targeting the mechanisms of cancer chemoresistance, either directly or indirectly, holds tremendous potential in oncology. These advances can facilitate patient stratification, detect acquired resistance during treatment, and provide noninvasive insights into tumor clonal heterogeneity. In this perspective, we will explore the various imaging approaches using PET, SPECT, and optical-based techniques, outlining the necessary steps for the future integration of molecular imaging in the study of cancer chemoresistance.
MOLECULAR BASIS OF CHEMORESISTANCE
The development of multidrug resistance (MDR) has not been fully clarified to date. Several factors are believed to play a role in the molecular mechanisms underlying resistance to antitumor drugs (2). Among those are mutations affecting the target of the chemotherapeutic drug, alterations in the transcellular drug efflux mechanism mediated by adenosine triphosphate (ATP)–binding cassette (ABC) transporters, enzymatic inactivations affecting the chemotherapeutic drug, abnormalities in the regulation of gene expression of DNA or RNA with consequent effects on protein production, remodeling of redox processes, and the influence of the cellular and noncellular tumor microenvironment (3).
Currently, overexpression of ABC transporters is considered the main factor responsible for MDR in tumor cells. Indeed, the forced extrusion of a chemotherapeutic drug from the tumor cell is one of the main mechanisms hindering oncologic therapies, as it reduces the drug concentration below effective levels. This mechanism is precisely determined by the activity of specific transporters belonging to the ABC pumps, which are also implicated in various serious diseases, including cystic fibrosis and several immune system disorders (4). The ABC transporters identified to date are 49. They are classified on the basis of gene sequence and structural similarities into 7 subfamilies, from ABCA to ABCG, or, as more recently indicated, from I to VII, based on the fold of their transmembrane domains (5). This transporter category is structurally characterized by the presence of transmembrane domains that identify and transport substrates (typically hormones, lipids, ions, or other hydrophobic and amphipathic molecules) and cytoplasmic domains that bind and hydrolyze ATP (adenosine triphosphate), enabling their function. Various hypotheses have been proposed regarding their mechanism and regulation, all sharing the following sequence of steps: ATP-dependent dimerization of the cytoplasmic domains, binding of substrates to the transmembrane domains, and consequent conformational change that allows the expulsion of the substrate. Among these, 3 transporters—ABCB1 (also known as P-glycoprotein, or P-gp), ABCG2 (breast cancer resistance protein), and ABCC1 (MDR-associated protein 1)—are certainly involved in MDR development (6). The human P-gp/ABCB1 transporter (a glycoprotein composed of 1,280 amino acids) was the first ABC transporter to be identified and is the most studied. Its overexpression in tumor cells of the pancreas, colorectal area, liver, adrenal glands, and kidneys is well known (7). Inhibiting the function of P-gp (through competitive or noncompetitive inhibition, interference with ATP hydrolysis, or alteration of lipids in the cell membrane) or activating its downregulation by targeting its upstream regulators are the 2 main current strategies for effectively reversing MDR mediated by this glycoprotein. Several synthetic approaches have been implemented to optimize drugs capable of performing these functions, but so far no single specific P-gp inhibitor has been successful in clinical trials (8).
ABC-TARGETED IMAGING
PET and SPECT radiopharmaceuticals for imaging ABC transporters, mainly P-gp, have been developed to provide insights into drug resistance in tumors and neurologic disorders by assessing P-gp function and expression (Fig. 1). These radiopharmaceuticals can be categorized into 3 main groups. The first is ABC substrates, which are compounds transported by the ABC transporters, allowing for the measurement of their activity and function. Since ABC transporters eject these radioactive substrates out of the cell, the tumor signals from imaging are inversely proportional to the expression levels of ABC transporters in cancer cells. To assess ABC activity in vivo, transporter inhibitors must be administered to detect changes in the radioactive signal. This approach can lead to false-positive results when the inhibitors alter ABC activity in the liver and kidneys, subsequently changing the pharmacokinetics of the radiolabeled substrates. Furthermore, many of these radiopharmaceuticals are substrates for multiple transporters, making it difficult to identify the specific ABC transporter responsible for MDR. The second main group of radiopharmaceutical is ABC transporter-inhibitors, which are based on the presence of an inhibitory function in the molecular structure of the radiopharmaceutical, such as the dimethoxytetrahydroisoquinoline moiety or stilbenyl moiety. These radiotracers can help evaluate ABC expression levels rather than its function, providing insight into how much of these transporters is present in specific tissues. The third group is radiolabeled P-gp–targeted antibodies, which are more effective for imaging P-gp than small-molecule substrates. Monoclonal antibodies have high specificity for recognizing P-gp, unlike radiopharmaceuticals, which may also target other ABC transporters. Additionally, the accumulation of these antibodies is directly proportional to P-gp expression levels, providing an added advantage. The main characteristics of the selected papers are summarized in Supplemental Table 2.
Overview of tracers used in ABC-targeted imaging.
SPECT Tracers
[99mTc]Tc-sestamibi is a lipophilic, monocationic compound formed by the coordination of 6 methoxyisobutylisonitrile ligands to the technetium (I) core (9). Since [99mTc]Tc-sestamibi is commercially available and widely used in clinical nuclear medicine for myocardial perfusion imaging and parathyroid scintigraphy, it may serve as an attractive tool for functional imaging of P-gp in excretory organs. [99mTc]Tc-sestamibi is a substrate for both ABCB1 and MDR-associated protein 1 (ABCC1). Its uptake and retention in tissues can indicate P-gp activity, providing valuable information about MDR in various cancers.
Recent data on this topic remain limited, partly because of the growing preference for PET-based imaging techniques. However, in 2021, Ghadi et al. explored itraconazole’s role as a P-gp inhibitor and its synergy with paclitaxel using [99mTc]Tc-sestamibi accumulation in HT-29 colorectal cancer tumor–bearing mice (10,11). They assessed cell viability by comparing treated and control cells, and in vivo tumor suppression was measured over 12 d. Itraconazole successfully blocked P-gp–mediated [99mTc]Tc-sestamibi efflux, increasing tracer accumulation in vitro. Coadministration with paclitaxel significantly enhanced cytotoxic effects, resulting in greater tumor suppression than monotherapy. Biodistribution studies confirmed increased tracer uptake in tumors and organs, supported by histopathologic findings. These results suggest that itraconazole boosts the antitumor efficacy of paclitaxel, and [99mTc]Tc-sestamibi might be a valuable radiotracer for evaluating treatment response in multidrug-resistant tumors.
It should be emphasized that in clinical practice, [99mTc]Tc-sestamibi has been used to image MDR in solid cancers (especially breast cancer) by comparing washout rates between the early phase (10–30 min) and the late phase (2–3 h) after the injection. Alongside [99mTc]Tc-sestamibi, the radiopharmaceutical [99mTc]Tc-tetrofosmin is widely used for myocardial perfusion studies and has recently been tested for imaging chemoresistance. Kobayashi et al. used a combination of in vitro and in vivo models to examine the efflux and metabolism of [99mTc]Tc-sestamibi and [99mTc]Tc-tetrofosmin (12). Human-derived cancer cell lines, including SK-N-SH (neuroblastoma), SK-MEL-28 (melanoma), and PC-3 (prostate cancer), were used to assess radiotracer uptake and efflux via ABC transporters such as MDR1 and MRP1–3. The researchers also conducted vesicle assays to test transporter activity and performed imaging on SK-N-SH–bearing mice to observe the whole-body distribution of the tracers. [99mTc]Tc-sestamibi was found to be rapidly exported by MDR1 and MRP1 transporters and metabolized within 30–60 min, leading to faster efflux and lower imaging reliability in late-phase scans. In contrast, [99mTc]Tc-tetrofosmin was stable, was exported by multiple transporters (MDR1, MRP1–3), and showed slower efflux, making it more suitable for both early-phase and late-phase imaging. This stability might allow for more accurate monitoring of drug resistance over time in clinical settings.
Using tricarbonyl technology, 2 novel 99mTc-based agents, [99mTc]Tc-TMEOP and [99mTc]Tc-DMEOP, were developed for myocardial imaging. In preclinical studies, [99mTc]Tc-TMEOP clearance was found to be mediated by P-gp, indicating its potential for the imaging of MDR tumors. Mendes’ group studied the uptake and efflux of [99mTc]Tc-DMEOP and [99mTc]Tc-TMEOP in prostate, lung, and breast cancer cells, including drug-resistant lines overexpressing P-gp (13). The effects of MDR inhibitors such as verapamil were also examined. In vivo experiments with xenograft tumor models confirmed MDR status through Western blot analysis. Both complexes exhibited similar uptake kinetics to [99mTc]Tc-sestamibi in human cancer cells, with uptake increasing over time. Notably, [99mTc]Tc-TMEOP uptake was significantly reduced in cells overexpressing P-gp but increased when verapamil was applied. In nude mice, [99mTc]Tc-TMEOP showed higher tumor uptake in MCF-7 xenografts than in MCF-7 tumors overexpressing P-gp.
Recently, Hernández-Lozano’s group compared the performance and sensitivity of [99mTc]Tc-sestamibi with 3 radiolabeled P-gp substrates for PET ([11C]N-desmethyl-loperamide, (R)-[11C]verapamil, and [11C]metoclopramide) in assessing P-gp function in the kidneys and liver of homozygous and heterozygous ABCB1a/b knockout mice, representing complete loss and moderately reduced P-gp expression, respectively (14). Mice were imaged using either dynamic PET or consecutive SPECT/CT scans, depending on whether they were injected with PET tracers or [99mTc]Tc-sestamibi. P-gp expression in the kidneys and liver was analyzed by immunofluorescence and Western blotting. Heterozygous ABCB1a/b mice exhibited intermediate P-gp levels compared with homozygous knockout or wild-type mice. Among the radiotracers examined, [99mTc]Tc-sestamibi performed best in measuring renal and hepatic P-gp function.
PET Tracers
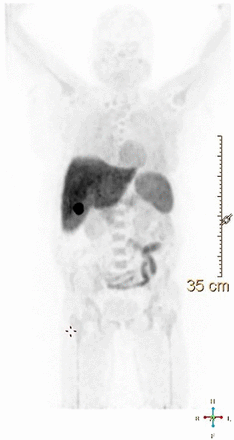
The tracer [18F]F-AVT-011 ([2-(4-{2-[6-(2-[18F]fluoroethoxy)-7-methoxy-3,4-dihydro-1H-isoquinolin-2-yl]ethyl}phenylcarbamoyl)-4,5-dimethoxyphenyl]amide) was developed by Kannan et al. in 2020 to measure ABC transporters’ function in tumors (15). It is synthesized through a nucleophilic reaction of cyclotron-produced [18F]fluoride with ethylene di(p-toluenesulfonate), followed by a reaction of [18F]fluoroethyl-tosylate with 6-O-desmethyl tariquidar. The study by Kannan et al. suggests that [18F]F-AVT-011 is likely a substrate for both ABCB1 (P-gp) and ABCG2, which are often coexpressed in MDR tumors. In mouse orthotopic tumor models, [18F]F-AVT-011 demonstrated lower uptake in malignancies with growing levels of P-gp expression. Additionally, it showed increased tumor uptake after the administration of tariquidar, a P-gp inhibitor. Considered as a whole, these results prompt [18F]F-AVT-011 as a substrate of ABC transporters, suitable for imaging P-gp function in vivo. The study’s major limitation was the low yield, likely due to the multistep radiolabeling process. In 2022, Kumar et al. synthesized a modified precursor to develop a single-step radiosynthesis protocol for [18F]F-AVT-011, achieving high yield and radiochemical purity (16). The high yield of [18F]F-AVT-011 is advantageous for supplying this tracer to distant hospitals without cyclotron facilities and enables further exploration of its applications in various disorders. In the same paper, the authors investigated the radiotracer biodistribution both in 12 mice and in 6 breast cancer patients. Human PET/CT imaging was performed 45–60 min after injection of 370 ± 40 MBq of [18F]F-AVT-011. The biodistribution was similar between mice and humans. A rapid radiotracer clearance from the blood pool was observed, followed by prevalent enterohepatic excretion (Fig. 2). At the PET scan, the higher radiotracer concentration was found in the gallbladder and in the liver, followed by spleen, colon, and myocardium. The correlation between the radiotracer uptake and the P-gp expression in the tumor was not explored in the published paper and will be the object of a future study. However, the authors report that low radiotracer uptake was detected in the breast lesions at PET imaging.
[18F]F-AVT-011 biodistribution in humans. %IA = percentage injected activity; p.i. = after injection. (Reprinted from (16); http://creativecommons.Org/licenses/by/4.0/.)
The [64Cu]Cu-DOTA-Pab-IR800 dual-modality PET/fluorescence probe was proposed by Wang et al. (17). This radiopharmaceutical is obtained by conjugating an anti–P-gp monoclonal antibody (Pab) and the fluorescent dye IR800 with DOTA, using amino groups as the binding sites. The resulting DOTA-Pab-IR800 was labeled with 64Cu, achieving a radiochemical yield of 30.4%. The specificity of the [64Cu]Cu-DOTA-Pab-IR800 probe was confirmed in vitro using P-gp–expressing 3T3-MDR1 cells and control 3T3 cells. Staining revealed a clear rightward shift in 3T3-MDR1 cells compared with controls, demonstrating specific binding to P-gp without compromising the antibody’s specificity. In vivo PET imaging using [64Cu]Cu-DOTA-Pab-IR800 in tumor-bearing mice showed significantly increased tumor-associated radioactivity compared with the [64Cu]Cu-DOTA-IgG control group, suggesting P-gp–mediated uptake. The 64Cu shorter half-life (12.7 h) compared with 111In or 131I makes it ideal for antibody imaging, reducing radiation exposure. Blood-pool activity remained high at 4 h after injection, suggesting 24–48 h as the optimal time for PET scans to achieve a favorable tumor-to-background ratio. Fluorescence imaging at 48 h after injection showed excellent tumor uptake. Notably, PET imaging revealed high liver uptake, whereas fluorescence imaging showed minimal signal, likely because of fluorescence’s limited sensitivity for deep tissues.
FUTURE DIRECTIONS
Recent oncologic research has focused on discovering new cancer drugs, but reliable tools for selecting the most effective treatment are still lacking in clinical practice. Imaging techniques that detect tumor chemoresistance could revolutionize treatment selection and advance personalized medicine. Indeed, many of the innovative molecularly targeted drugs, such as PARP inhibitors used in ovarian and prostate cancer (e.g., olaparib and rucaparib), are substrates of ABC transporters. However, the discussion of these drugs is beyond the scope of this paper, which focuses on cytotoxic chemotherapeutic agents. The imaging of resistance to molecularly targeted drugs should be the subject of further investigation (18).
Molecular imaging targeting ABC transporters is still in its early stages, but its potential is clear. Most studies use radiolabeled substrates of P-gp and other ABC transporters for SPECT or PET imaging. [99mTc]Tc-sestamibi has been widely used in nuclear medicine and is known for being a substrate of ABC transporters enabling visualization of MDR through sequential imaging at early and late time points (12). The newer radiotracer [99mTc]Tc-TMEOP has the same mechanism as for [99mTc]Tc-sestamibi, being another substrate of ABC transporters (13). However, head-to-head comparison of the 2 radiotracers for visualizing MDR is still lacking in the literature. SPECT imaging is widely available worldwide. Still, it is impaired by low spatial resolution in comparison to PET imaging. In this regard, new multiple-field-of-view SPECT/CT devices and solid-state detectors improve sensitivity, energy resolution, and image contrast, addressing some limitations of traditional SPECT imaging (19).
Several PET radiotracers have been investigated as substrates of ABC transporters, including [11C]N-desmethyl-loperamide, (R)-[11C]verapamil, and [11C]metoclopramide. However, these radiotracers are all hindered by low availability, being labeled with [11C] and thus requiring an in loco cyclotron. In a couple of preliminary studies, the new PET radiotracer, [18F]F-AVT-011, demonstrated to be a promising probe for in vivo visualization of P-gp–related chemoresistance (15,16). Moreover, this PET radiotracer could guarantee a wider diffusion in daily clinical practice, since it is labeled with 18F. However, initial data suggest that [18F]F-AVT-011 may not be feasible to study chemoresistance in every type of cancer. Its prevalent enterohepatic excretion could hamper the characterization of chemoresistance related to ABC transporters in cancers of the abdominal district. Conversely, as reported by the authors, this radiotracer could be suitable for revealing chemoresistance in breast cancer, pending confirmatory large-cohort studies. Notably, radiolabeled substrates of ABC transporters may require use alongside other imaging techniques, like PET/CT with [18F]FDG, to assess tumor metabolism and MDR. Total-body PET/CT scanners could enable dual-tracer protocols while reducing radiation dose. For example, Liu et al. successfully implemented a 1-stop protocol using ultra-low-dose [18F]FDG (0.37 MBq/kg) with a 10-min scan, followed by a dynamic scan with half-activity (0.925 MBq/kg) of [68Ga]Ga-FAPI-04 (5,20).
Data on ABC transporter inhibitors are still preliminary. [18F]F-MC225 has shown good pharmacokinetics, high sensitivity, and low radiometabolite levels, making it a promising radiotracer for assessing P-gp function in the human brain (21). However, to date no studies have assessed the potential of [18F]F-MC225 for imaging cancer chemoresistance.
Radiolabeled P-gp antibodies may represent a valid option for revealing cancer chemoresistance in the future. Their main advantage is high specificity, and their main limits are elevated costs and suboptimal timing for imaging acquisition. [64Cu]Cu-DOTA-Pab-IR800 has been investigated with promising preliminary results in a study by Wang et al. (17). The radiotracer revealed chemoresistant tumors with a favorable tumor-to-background ratio 48 h after injection. The same probe was found feasible for fluorescence imaging, with high tumor specificity. In patients with prevalent chemosensitive metastatic disease, a selective fluorescence-guided surgical approach of chemoresistant metastases—previously diagnosed through PET imaging—could be pursued. From this perspective, optical imaging in the field of chemoresistance has been only minimally explored and, especially in combination with radionuclide imaging, represents an area worthy of further investigation.
Recently, many classic cytotoxic compounds are being repurposed as antibody–drug conjugates’ payloads because of their well-characterized mechanisms of action and toxicologic profiles (22). However, resistance mechanisms such as decreased antigen expression, tumor heterogeneity, and ABC transporters, which expel payloads via efflux pumps, can reduce antibody–drug conjugate efficacy. In this perspective, ABC-targeted imaging might be of value to identify acquired resistance to therapeutic approaches mediated by antibody–drug conjugates. Further consideration should be given to the lack of correlation, observed in both preclinical and clinical studies, between uptake of various radiopharmaceuticals used and expression of ABC transporters. Although in most of the studies MDR status was determined using different methods (immunostaining, Western blot, polymerase chain reaction, etc.), no correlation was established between the level of uptake and the degree of ABC transporter expression. This issue should be addressed in future studies.
Last, radiolabeled tetraphenylphosphonium compounds, particularly [64Cu]Cu-labeled phosphonium cations, have been used in past years for imaging chemoresistance and could rise to prominence again (23). However, their application is limited by suboptimal tumor selectivity, significant background uptake in nontarget tissues, and inconsistencies in biodistribution and metabolic stability. To enhance their utility for chemoresistance imaging, efforts should focus on optimizing bifunctional chelators and radiometal chelates to improve the biokinetics and biodistribution properties of these compounds. With an eye toward the future, imaging of targeting ABC transporters might become a valuable tool for revealing tumor chemoresistance, assisting oncologists in selecting effective chemotherapeutic agents. [18F]F-AVT-011 and [64Cu]Cu-DOTA-Pab-IR800 are promising PET agents for this purpose. However, they remain experimental radiotracers with only preliminary evidence published in the literature, whereas current imaging of chemoresistance in daily clinical practice is still limited to conventional SPECT radiotracers, particularly [99mTc]Tc-sestamibi. As we deepen our understanding of chemoresistance, the development of innovative imaging agents has the potential to transform cancer treatment by facilitating tailored therapeutic strategies that effectively address resistance mechanisms.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Feb. 6, 2025.
- © 2025 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 14, 2024.
- Accepted for publication January 14, 2025.