Abstract
242541
Introduction: As the use of radiopharmaceuticals grows, safe and effective patient management with respect to radiation risk to individuals around the patient is critical. Patient release compliant with applicable regulations is, of course, essential. This involves placing time and distance limits on patient interaction post-radiopharmaceutical administration, to maintain safety of patients’ family members and the general. These contact limitation periods should be customized for each patient, and straightforward for clinical physicists to evaluate and patients to follow in a standardized way. MIRDrelease is a patient-release calculation worksheet that uses the release criteria methodology promulgated in the NCRP 115 [1]. MIRDrelease is freely accessible at mirdsoft.org.
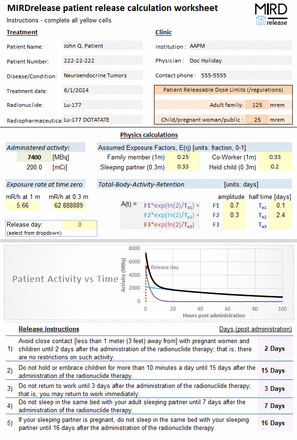
Methods: The MIRDrelease tool is built in the widely accessible Microsoft Excel platform and has an easy-to-use, interactive graphical user interface. MIRDrelease consists of a Microsoft Excel Spreadsheet that contains two tabs. The 'Patient Release' tab serves as the input/output single page graphical user interface and summarizes all calculations to be used in generating the release instructions. The 'Engine' tab is a revised version of the NCRP 155 patient release spreadsheet; it operates as the data processor and utilizes the equations from Appendix A of NCRP 155. This tab transparently allows users to inspect all calculations.
In MIRDrelease there are currently five exposure scenarios reported to the user to support release instructions:
• How many days post-administration should the patient avoid close contact with pregnant women and children, after which time there would be no restrictions?
• How many days post-administration should the patient avoid holding or embracing children more than 10 minutes per day? This further requires that the patient to avoid close contact with these children altogether during the first day post-administration of the radiopharmaceutical therapy.
• How many days post-administration should the patient avoid going to work so as not to expose co-workers?
• How many days post-administration should the patient avoid sleeping in the same bed with his/her sleeping partner? This assumes that your sleeping partner is not pregnant; f the patient’s sleeping partner is pregnant, refer to scenario 5.
• How many days post-administration should the patient avoid sleeping in the same bed with their pregnant sleeping partner if they are pregnant? This further requires that the patient avoid contact with his/her pregnant sleeping partner altogether during the first day post-radiopharmaceutical therapy administration.
Results: MIRDrelease supports radiopharmaceutical therapy by providing standardized and tailored calculations and instructions for patients’ release after radionuclide therapy. Whole-body retention parameters used in the spreadsheet can be adjusted to reflect patient specific dose rate measurements, or population based radiopharmaceutical biokinetics (a conservative assessment of the whole-body retention function). The sheet provides dynamically-generated instructions in the mentioned exposure scenarios. Additionally, any radiopharmaceuticals can be incorporated into the spreadsheet, by providing their retention functions.
Conclusions: Written instructions to reduce radiation exposure to the public and family should be given to patients undergoing nuclear medicine therapy before they are released from the hospital. A cautious approach to guaranteeing public radiation safety is crucial for both public safety as well as satisfying regulations. MIRDrelease offers a user-friendly interface and utilizes a pragmatic and standardized approach for calculation and specification of patient release instructions. With time, MIRDrelease could help in harmonizing the diverse regulatory and practice frameworks that exist. It also provides an essential information and instructions that must be communicated to the patient and family to allow release after radiopharmaceutical therapy.