Abstract
242324
Introduction: Multiple System Atrophy (MSA) is a neurodegenerative disease marked by prominent alpha-synuclein (α-Syn) pathology, leading to multisystem neuronal and oligodendrocyte degeneration. Clinically, it presents with severe autonomic failure, orthostatic hypotension, neurogenic bladder, and sexual dysfunction, coupled with parkinsonism and/or ataxia. Like Parkinson's disease (PD), the loss of nigrostriatal dopamine neurons is believed to trigger parkinsonian motor signs in MSA. However, MSA patients exhibit a limited response to dopaminergic drugs, suggesting changes beyond the presynaptic dopaminergic nerve terminal . Prior work from our group has shown evidence of cholinergic changes in MSA using a spatially less resolute radioligand (Gilman et al. 2010; PMID: 20439843). In this study, we used SPM voxel-based morphometric analysis of the spatially resolute [18F]-FEOBV vesicular acetylcholine transporter (VAChT) brain PET ligand to identify the topography of cholinergic vulnerability in MSA as compared to normal controls.
Methods: 7 MSA (Gender: 4/3 Male/Female; Age: 63 ± 7.45; MoCA: 24.5 ± 3.62; Hoehn and Yahr: 3.08 ± 0.82; MDS-UPDRS III: 50.64 ± 20.28) subjects & 22 NC (Gender: 10/22 Male/Female; Age: 70.73 ± 7.84; MoCA: 28.14 ± 1.36) underwent brain VAChT ([18F] FEOBV) PET and MR imaging. Most patients had the MSA-P subtype. Voxel-based t-test comparisons between the MSA patients and controls were performed on SPM12. Clusters surviving the multiple comparisons using cluster-level false discovery rate (FDR 0.05) correction were defined as significant.
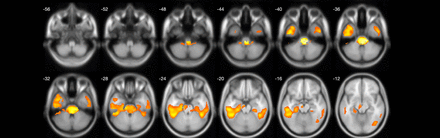
Results: Compared to the control, the whole brain voxel-based t-tests showed a significant reduction in cholinergic activities present in the left more than right fusiform, parahippocampal gyrus, left more than right inferior temporal lobes, pons, upper medulla and the left lobules IV, V, VI and IX of cerebellar hemisphere (FDR-corrected at 0.05).
Conclusions: Unlike our prior observation our study demonstrate most prominent but more limited brainstem changes in the pons and more limited left sided cerebellar reductions. Novel observations include the left more than right fusiform and ParaHippocampal, temporal lobe changes. Notable absent were striatal binding changes. The specific topography of the cholinergic vulnerability in MSA may be another factor explaining treatment refractoriness to dopaminergic medications. These findings may augur novel cholinergic and/or neurostimulation approaches to treat this disabling parkinsonian disorder.
Funding: Study funded by National Institutes of Health (P01 NS015655, RO1 NS070856, P50 NS091856, P50 NS123067), Department of Veterans Affairs grant (I01 RX001631), the Michael J. Fox Foundation, and the Parkinson’s Foundation.