Abstract
242184
Introduction: The limited spatial resolution of positron emission tomography (PET) images can reduce their quantitative and diagnostic accuracy. Both physical and post-processing factors contribute to this low resolution. Deep learning models, especially deep generative models, have seen successful at improving the quality of PET images. Building on the increasing popularity of diffusion probabilistic models (DPMs) for image generation tasks, we propose an approach to generate vivid, high-resolution (HR) tau PET images by iterative refinement, using a DPM model for super-resolution (SR) conditioned on a low-resolution (LR) tau PET input. SR PET is particularly significant in the imaging of tau tangles in Alzheimer’s disease due to the localized nature of its aggregation in early disease stages. Whereas DPMs have previously been used for PET denoising, to our knowledge, this is the first application of DPMs for SR PET and for tau PET imaging.
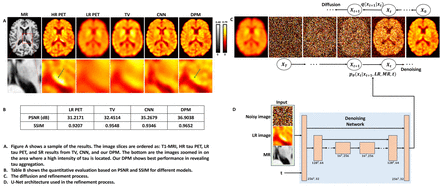
Methods: A DPM is a parameterized Markov chain designed to produce samples that match a desired data distribution through a finite number of refinement steps. The refinement is learned by reversing a diffusion process, a Markov chain that gradually adds noise to the data until the signal is destroyed. Given paired LR and HR tau PET images, the diffusion model can take the LR image as an additional input, to serve as guidance of the refinement process, so that the model is able to produce an HR image, with the same content as the LR input. Compared to CNN-based SR models, diffusion models can learn much richer data distributions and generate vivid images. The model we implemented is based on the U-Net architecture with a self-attention mechanism. Five sets of tau PET scans from a GE DMI scanner, co-registered with T1-MRI images are used for training, while 4 of such sets are used for evaluation. The scanning protocol involved image acquisition for 75 to 105 min. time window after a 370 MBq 18F-Flortaucipir bolus injection. PET data were stored in list mode and reconstructed as 6 × 5 minutes frames using ordered subset expectation maximization (OSEM). The original PET scans are used as ground truth HR images, and we generated paired LR images by applying a Gaussian filter with a full width at half maximum (FWHM) of 6 mm. The MRI scans are used in conjunction with the LR PET as input, providing HR anatomical information. We used a total of 2,000 refinement steps for the diffusion and denoising processes and deployed a linear noise schedule, where the variance of the Gaussian noise used in each step grows from 10-6 to 10-2. In each training step, the model takes 4 inputs: the time step t, the noisy image xt+1, which is sampled from a standard Gaussian distribution and denoised for 2,000-t steps, the LR PET image and the MR image. The model was trained to predict the noise that was added to the HR PET in the diffusion process, which could then be used to further denoise the image. An L1 loss function was computed using the actual noise according to the noise schedule as ground truth. Our model was trained for 420,000 steps.
Results: Peak signal-to-noise ratio (PSNR) and structural similarity index measure (SSIM) were used as reference-based evaluation metrics. For comparison, we implemented a CNN-based end-to-end SR model trained on the same datasets and a total variation(TV)-penalized deconvolution approach. The mean PSNR order with corresponding mean SSIM values was as follows: DPM (36.90dB/0.97) > CNN (35.27dB/0.93) > TV (32.45dB/0.95). Among the tested approaches, our proposed diffusion model achieved best PSNR and SSIM values.
Conclusions: We proposed a DPM to perform SR on tau PET images by iterative refinement. The model can produce rich-detailed, HR PET images. Our results show that the diffusion model outperformed both CNN-based and TV-penalized deconvolution approach on our dataset.