Abstract
241971
Introduction: Cisplatin (CDDP) is a frontline chemotherapy drug in clinical cancer treatment, but its nephrotoxicity is a major limiting factor for chemotherapy effectiveness.
Currently, there is no specific medication for cisplatin-induced renal toxicity in clinical practice. Consequently, designing and developing a combination drug delivery method that does not affect the therapeutic effect of CDDP but can absorb or capture CDDP molecules in the kidney is expected to inhibit CDDP 's direct damage to renal tissue, mitigate its toxicity, and maximize its anti-tumor efficacy.
We planned to construct DNA nanostructures (DNS) capable of loading CDDP. Through the enhanced permeability and retention (EPR) effect, these nanostructures can accumulate in tumors after injection into the body, verified by PET-CT imaging results. Simultaneously, considering the complex tumor microenvironment, these DNS gradually disintegrate, releasing CDDP drugs. The disintegrated DNA chains possess specific renal targeting ability in the body and, therefore, serve as exogenous DNA, enabling the direct capture of CDDP molecules in the kidneys. This approach aims to alleviate CDDP -induced kidney damage while preserving its anti-tumor efficacy.
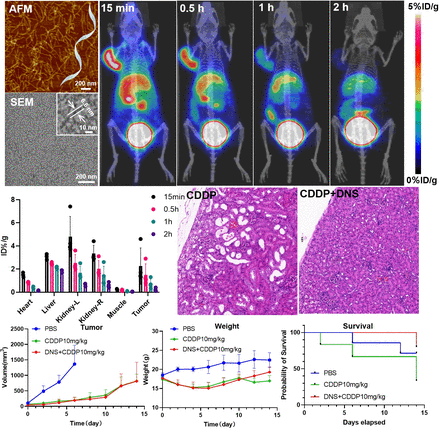
Methods: Through an annealing process, we successfully synthesized DNS. Subsequently, after the successful synthesis of DNS, we labeled it with the Ga-68 isotope for PET-CT imaging. Following the imaging results, we conducted a therapeutic experiment by preparing a mixed injection solution combining DNS and CDDP, which was then administered to tumor-bearing mice via tail vein injection.
Results: Using PET-CT imaging, we found that DNS accumulates rapidly in tumors, reaching 3.7 %ID/g in 15 min. The mixed injection solution, administered at a CDDP dose of 10 mg/kg, was delivered via tail vein injection in tumor-bearing mice. Tumor volume, body weight, and survival rates were continuously monitored for two weeks. Our observations revealed that the tumor growth trend in the DNS-CDDP treatment group showed no significant difference compared to the CDDP group. However, when compared to the PBS treatment group, the tumor growth rate was markedly inhibited. Both the DNS-CDDP and CDDP groups experienced a decrease in body weight in the first four days due to the systemic toxicity of cisplatin, followed by a gradual increase in body weight thereafter. Encouragingly, the tumor-bearing mice in the DNS-CDDP group exhibited a higher survival rate after 14 days compared to both the CDDP and PBS groups. Histopathological staining (HE staining) and biochemical analyses provided evidence supporting the protective role of DNA on the kidneys.
Conclusions: The addition of DNA nanomaterial structures to cisplatin injection did not impact the inhibitory effect of cisplatin on tumor growth. However, it significantly increased the survival rate of tumor-bearing mice. This experiment offers new insights and methods for mitigating the renal toxicity associated with clinical applications of cisplatin, with potential applications in clinical treatment.