Abstract
241233
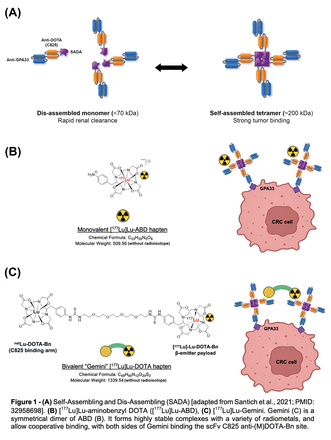
Introduction: Three-step pretargeted radioimmunotherapy (PRIT) using (1) IgG based bispecific antibody (BsAb) targeting the glycoprotein A33 (GPA33), (2) dendrimer clearing agent, and (3) a theranostic monovalent radioligand [177Lu]Lu-2-(4-aminobenzyl)-DOTA ([177Lu]Lu-ABD), has shown cures with minimal toxicities against subcutaneous human colorectal cancer (CRC) xenografts. [177Lu]Lu-Gemini (Fig. 1C) consisting of 2 benzyl-DOTA chelator arms connected by a PEG4 linker, was developed to increase tumor uptake and tumor avidity by slowing down off-rate of tumor-bound BsAb/radiohapten complexes. With the advent of a 2-step approach for enhanced therapeutic indexes (TI) eliminating the need of any clearing agent, Self-Assembling and Dis-Assembling (SADA) (PMID: 32958698) PRIT has exceptional promise (Fig. 1A). Here we compare the bivalent Gemini radiohapten with the standard monovalent radioligand in GPA33-SADA PRIT (Fig. 1B).
Methods: The bivalent radiohapten Gemini was synthesized by linking two S-2-(4-isothiocyanatobenzyl)-DOTA (p-SCN-Bn-DOTA) chelators via a 1,14-diamino-PEG4linker. [177Lu]Lu-Gemini was prepared with no carrier added 177LuCl3 to a molar activity of 123 MBq/nmol and radiochemical purity of >99%. [177Lu]Lu-Gemini SADA specificity was verified in vitro. Disassembling of affinity purified SW1222-SADA was studied by mass photometry. Groups (n= 4-5 mice/group) of SW1222-tumor bearing mice (average tumor volume: 230 mm3) were injected intravenously in the tail vein: 250 µg (1.19 nmol) of anti-GPA33 SADA BsAb, followed 72h later with [177Lu]Lu-ABD (500 μCi/18.5 MBq, 400 pmol) or ([177Lu]Lu-Gemini (1000 μCi/37 MBq, 200 pmol). Ex vivo biodistribution of [177Lu]Lu-Gemini was compared to [177Lu]Lu-ABD, 2h and 120h after payload. Anti-GPA33 SADA-PRIT therapeutic studies comparing [177Lu]Lu-Gemini versus [177Lu]Lu-ABD are currently ongoing.
Results: By mass photometry, GPA33-SADA disassembled in vitro to dimers in the presence of Lu-Gemini instead of to only monomers with Lu-ABD. By ex vivo biodistribution (Fig. 2), efficient tumor targeting was noted with both radiohaptens, achieving a tumor activity of 19.4±8.1 %ID/g (n=5) 2h after [177Lu]Lu-Gemini SADA-PRIT, and 15.6±5.6 %ID/g (n=5) after [177Lu]Lu-ABD SADA-PRIT. At 2h post-radioligand injection, tumor-to-tissue ratios for [177Lu]Lu-Gemini were 42 and 7 for blood and kidneys respectively, and for [177Lu]Lu-ABD, 83 and 11. By day 5 (120h), ex vivo biodistribution revealed higher tumor retention of [177Lu]Lu-Gemini (4.9±2.6 %ID/g, n=4) compared to [177Lu]Lu-ABD (1.1±0.4 %ID/g, n=4), with tumor-to-tissue ratios of 247 and 9 for blood and kidneys respectively for [177Lu]Lu-Gemini, versus 224 and 4 for [177Lu]Lu-ABD. The tumor, blood, and kidney approximated absorbed doses (Fig. 2C) were respectively 2072 mGy/MBq, 20 mGy/MBq (TI= 104) and 271 mGy/MBq (TI= 7.6) for [177Lu]Lu-Gemini; and 1018 mGy/MBq, 17 mGy/MBq (TI= 60), and 136 mGy/MBq (TI= 7.5) for [177Lu]Lu-ABD.
Conclusions: These studies showed that bivalent [177Lu]Lu-Gemini improved tumor uptake and tumor retention without comprising TIs when used in GPA33-SADA PRIT. The first-in-human [177Lu]Lu-DOTA SADA-PRIT phase 1 clinical trial (targeting GD2) (NCT05130255) has confirmed safety of the SADA system. The clinical translation of bivalent versus monovalent ligands for SADA-PRIT, especially when applied to GPA33(+) CRC seems prudent.