Abstract
241209
Introduction: Radiotherapy combined with immune checkpoint blockade holds great promise for synergistic antitumor efficacy. Targeted radionuclide therapy delivers radiation directly to tumor sites. LNC1004 is a fibroblast activation protein (FAP)-targeting vector conjugated with the albumin binder Evans Blue for enhanced tumor uptake and retention. Combining radionuclide-targeted therapy against pancancer targets with immunotherapy may be a promising treatment strategy for certain advanced tumors. Moreover, single-cell RNA-sequencing (scRNA-seq) technology offers a comprehensive view of cellular and molecular interactions at an unprecedented resolution. However, to date, no studies have reported the application of scRNA-seq in radionuclide-targeted or combined immunotherapies.
Methods: We investigated the therapeutic efficacy of 177Lu-LNC1004 in combination with PD-L1 in a preclinical setting. We pioneered the use of scRNA-seq for analyzing the changes within the TME and elucidated the underlying mechanisms of action of this combination treatment. Additionally, we assessed the safety and efficacy of 177Lu-LNC1004 in a small cohort of patients (clinical trial: NCT05963386) with various cancer types and analyzed the abundance of immune cell types among peripheral blood mononuclear cells (PBMCs) pre- and posttreatment.
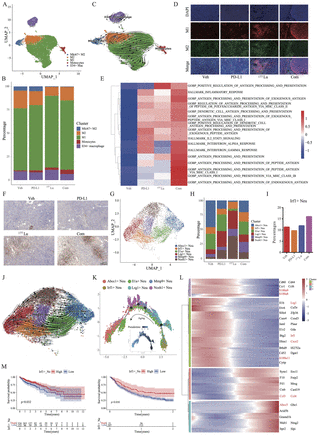
Results: 177Lu-LNC1004 stimulation increased tumor PD-L1 expression both in vitro and in vivo. We further explored the antitumor efficacy of a combination treatment including anti-PD-L1 antibody and 177Lu-LNC1004 radioligand therapy. Combination therapy led to complete eradication of all tumors in MC38/NIH3T3-FAP mixed tumor xenografts, with mice showing 100 % tumor rejection upon rechallenge. The combination therapy reprogrammed the tumor microenvironment in mice to foster antitumor immunity by suppressing malignant progression and increasing cell-to-cell communication, CD8+ T-cell activation and expansion, M1 macrophage counts (Figure 1A-E), antitumor activity of neutrophils (Figure 1F-L), and TCR diversity. A preliminary clinical study demonstrated that 177Lu-LNC1004 was well-tolerated and effective in patients with refractory cancers, resulting in an increase in antigen processing and presentation juxtaposed with T-cell inactivation (Figure 2).
Conclusions: In conclusion, our preclinical data suggested that 177Lu-LNC1004 can amplify the antitumor efficacy of ICB. Furthermore, preliminary clinical data indicated that 177Lu-LNC1004 is a safe and well-tolerated therapeutic regimen with encouraging antitumor activity. Our data foster further exploration of the synergy between 177Lu-LNC1004 and immunotherapy in patients with advanced and refractory disease, particularly in those with FAP-positive tumors.